Chemistry:Aescin
| |
| Names | |
|---|---|
| IUPAC name
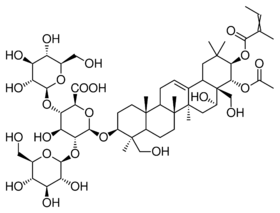
β-D-Glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-(22α-(acetyloxy)-16α,24,28-trihydroxy-21β-{[(2Z)-2-methylbut-2-enoyl]oxy}olean-12-en-3β-yl β-D-glucopyranosiduronic acid)
| |
| Systematic IUPAC name
(2S,3S,4S,5R,6R)-6-{[(3S,4S,4aR,6aR,6bS,8R,8aR,9R,10R,12aS,14aR,14bR)-9-(Acetyloxy)-8-hydroxy-4,8a-bis(hydroxymethyl)-4,6a,6b,11,11,14b-hexamethyl-10-{[(2Z)-2-methylbut-2-enoyl]oxy}-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-yl]oxy}-4-hydroxy-3,5-bis{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2-carboxylic acid | |
| Other names
Escin
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C55H86O24 | |
| Molar mass | 1131.269 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aescin or escin is a mixture of saponins with anti-inflammatory, vasoconstrictor and vasoprotective effects found in Aesculus hippocastanum (the horse chestnut). Aescin is the main active component in horse chestnut, and is responsible for most of its medicinal properties. The main active compound of aescin is β-aescin, although the mixture also contains various other components including α-aescin, protoescigenin, barringtogenol, cryptoescin and benzopyrones.[1]
Evidence suggests that aescin, especially pure β-aescin, is a safe and effective treatment for short-term treatment of chronic venous insufficiency;[2][3] however, more high quality randomized controlled trials are required to confirm the effectiveness.[3] Horse chestnut extract may be as effective and well tolerated as the use of compression stockings.[3]
Mechanism of action
Aescin appears to produce effects through a wide range of mechanisms. It induces endothelial nitric oxide synthesis by making endothelial cells more permeable to calcium ions, and also induces release of prostaglandin F2α.[4][5][6] Other possible mechanisms include serotonin antagonism and histamine antagonism and reduced catabolism of tissue mucopolysaccharides.[4]
References
- ↑ Ramelet, Albert-Adrien (24 November 2016). "Venoactive Drugs". Sclerotherapy: treatment of varicose and telangiectatic leg veins (6th ed.). Elsevier Science Health Science. pp. 426–434. ISBN 978-0-323-37726-3.
- ↑ Goldman, Mitchel P. (2016). Sclerotherapy : treatment of varicose and telangiectatic leg veins. Weiss, Robert A.,, Guex, Jean-Jerome (6th ed.). Amsterdam: Elsevier Science Health Science. ISBN 978-0-323-37727-0. OCLC 959274899.
- ↑ 3.0 3.1 3.2 Pittler, Max H.; Ernst, Edzard (2012-11-14). "Horse chestnut seed extract for chronic venous insufficiency". The Cochrane Database of Systematic Reviews 11 (11): CD003230. doi:10.1002/14651858.CD003230.pub4. ISSN 1469-493X. PMID 23152216.
- ↑ 4.0 4.1 Sirtori CR (September 2001). "Aescin: pharmacology, pharmacokinetics and therapeutic profile". Pharmacol. Res. 44 (3): 183–193. doi:10.1006/phrs.2001.0847. PMID 11529685.
- ↑ "Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta". Vascul. Pharmacol. 47 (1): 68–73. July 2007. doi:10.1016/j.vph.2007.04.003. PMID 17512261.
- ↑ "The mode of action of aescin and the release of prostaglandins". Prostaglandins 14 (2): 241–249. August 1977. doi:10.1016/0090-6980(77)90169-1. PMID 897216.
External links
- Information on horse chestnut extract from Memorial Sloan-Kettering Cancer Center
- CID 76967409 from PubChem - alpha-Aescin
 |

