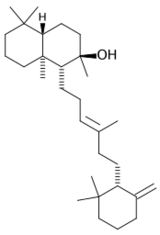
Chemistry:Ambrein
| |
| Names | |
|---|---|
| IUPAC name
(1R,2R,4aS,8aS)-1-{(3E)-6-[(1S)-2,2-dimethyl-6-methylidenecyclohexyl]-4-methylhex-3-en-1-yl}-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol
| |
| Other names
Ambrein
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H52O | |
| Molar mass | 428.745 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ambrein is a triterpene alcohol that is the chief constituent of ambergris, a secretion from the digestive system of the sperm whale, and has been suggested as the possible active component producing the supposed aphrodisiac effects of ambergris.[1] Although ambrein itself is odorless, it serves as the biological precursor for a number of aromatic derivatives such as ambroxan and is thought to possess fixative properties for other odorants.
It has been shown to act as an analgesic[2] and it has been proven to increase sexual behavior in rats,[3] providing some support for its traditional aphrodisiac use.
Apart from its supposed aphrodisiac effects, ambrein has been shown to decrease spontaneous contractions of smooth muscles in rats, guinea pigs, and rabbits. It is able to reduce these contractions by serving as an antagonist and interfering with the Ca2+ ions from outside of the cell.[4]
Discovery
In 1946, Ruzicka and Lardon "established that the fragrance of ambergris is based on the triterpene (named) ambrein".[5][6][7]
Biosynthesis
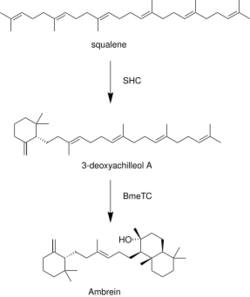
Ambrein is synthesized from common triterpenoid precursor squalene. The squalene-hopene cyclase (SHC) catalyzes cyclization of squalene into the monocyclic 3-deoxyachilleol A. Tetraprenyl-beta-curcumene synthase (BmeTC) converts 3-deoxyachilleol A into the tricyclic ambrein.[8]
References
- ↑ "Aphrodisiacs past and present: a historical review". Clinical Autonomic Research 11 (5): 303–7. October 2001. doi:10.1007/BF02332975. PMID 11758796.
- ↑ "Studies on the mode of action of ambrein as a new antinociceptive compound". Japanese Journal of Pharmacology 60 (2): 67–71. October 1992. doi:10.1254/jjp.60.67. PMID 1479744.
- ↑ "Effect of ambrein, a major constituent of ambergris, on masculine sexual behavior in rats". Archives Internationales de Pharmacodynamie et de Therapie 329 (2): 283–94. 1995. PMID 8540767.
- ↑ "Effect of ambrein on smooth muscle responses to various agonists". Journal of Ethnopharmacology 60 (1): 19–26. February 1998. doi:10.1016/s0378-8741(97)00126-8. PMID 9533428.
- ↑ Ruzicka, L.; Lardon, F. (1946). "Zur Kenntnis der Triterpene. (105. Mitteilung) Über das Ambreïn, einen Bestandteil des grauen Ambra". Helvetica Chimica Acta 29 (4): 912–921. doi:10.1002/hlca.19460290414.
- ↑ Prelog, Vladimir; Jeger, Oskar (1980). "Leopold Ruzicka (13 September 1887 – 26 September 1976)". Biogr. Mem. Fellows R. Soc. 26: 411–501. doi:10.1098/rsbm.1980.0013.
- ↑ Hillier, Stephen G.; Lathe, Richard (2019). "Terpenes, hormones and life: Isoprene rule revisited". Journal of Endocrinology 242 (2): R9–R22. doi:10.1530/JOE-19-0084. PMID 31051473.
- ↑ "Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli". Biotechnology Letters 40 (2): 399–404. February 2018. doi:10.1007/s10529-017-2483-2. ISSN 1573-6776. PMID 29204767.
 |