Chemistry:Squalene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6E,10E,14E,18E)-2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1728919 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | Squalene |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C30H50 | |
| Molar mass | 410.730 g·mol−1 |
| Appearance | Colourless oil |
| Density | 0.858 g·cm−3 |
| Melting point | −5 °C (23 °F; 268 K)[4] |
| Boiling point | 285 °C (545 °F; 558 K) at 3.3 kPa[2] |
| log P | 12.188 |
Refractive index (nD)
|
1.4956 (at 20 °C) [3] |
| Viscosity | 12 cP (at 20 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 110 °C (230 °F; 383 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as Squalus is a genus of sharks). An estimated 12% of bodily squalene in humans is found in sebum.[5] Squalene has a role in topical skin lubrication and protection.[6]
Most plants, fungi, and animals produce squalene as biochemical precursor in sterol biosynthesis, including cholesterol and steroid hormones in the human body.[7][8][9] It is also an intermediate in the biosynthesis of hopanoids in many bacteria.[10]
Squalene is an important ingredient in some vaccine adjuvants: The Novartis and GlaxoSmithKline adjuvants are called MF59 and AS03, respectively.[11]
Role in triterpenoid synthesis
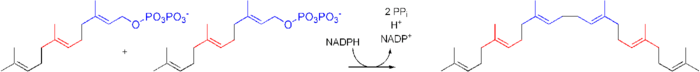
Squalene is a biochemical precursor to both steroids and hopanoids.[12] For sterols, the squalene conversion begins with oxidation (via squalene monooxygenase) of one of its terminal double bonds, resulting in 2,3-oxidosqualene. It then undergoes an enzyme-catalysed cyclisation to produce lanosterol, which can be elaborated into other steroids such as cholesterol and ergosterol in a multistep process by the removal of three methyl groups, the reduction of one double bond by NADPH and the migration of the other double bond.[13] In many plants, this is then converted into stigmasterol, while in many fungi, it is the precursor to ergosterol.[citation needed]
The biosynthetic pathway is found in many bacteria,[14] and most eukaryotes, though has not been found in Archaea.[15]
Production
Biosynthesis
Squalene is biosynthesised by coupling two molecules of farnesyl pyrophosphate. The condensation requires NADPH and the enzyme squalene synthase.
Industry
This section is missing information about all routes currently used; market share of each. (January 2022) |
Synthetic Squalene is prepared commercially from geranylacetone.[16]
Shark conservation
In 2020, conservationists raised concerns about the potential slaughter of sharks to obtain squalene for a COVID-19 vaccine.[17]
Environmental and other concerns over shark hunting have motivated its extraction from other sources.[18] Biosynthetic processes use genetically engineered yeast or bacteria.[19][20]
Uses
As an adjuvant in vaccines
Immunologic adjuvants are substances, administered in conjunction with a vaccine, that stimulate the immune system and increase the response to the vaccine. Squalene is not itself an adjuvant, but it has been used in conjunction with surfactants in certain adjuvant formulations.[11]
An adjuvant using squalene is Seqirus' proprietary MF59, which is added to influenza vaccines to help stimulate the human body's immune response through production of CD4 memory cells. It is the first oil-in-water influenza vaccine adjuvant to be commercialised in combination with a seasonal influenza virus vaccine. It was developed in the 1990s by researchers at Ciba-Geigy and Chiron; both companies were subsequently acquired by Novartis.[11] Novartis was later acquired by CSL Bering and created the company Seqirus. It is present in the form of an emulsion and is added to make the vaccine more immunogenic.[11] However, the mechanism of action remains unknown. MF59 is capable of switching on a number of genes that partially overlap with those activated by other adjuvants.[21] How these changes are triggered is unclear; to date, no receptors responding to MF59 have been identified. One possibility is that MF59 affects the cell behaviour by changing the lipid metabolism, namely by inducing accumulation of neutral lipids within the target cells.[22] An influenza vaccine called FLUAD which used MF59 as an adjuvant was approved for use in the US in people 65 years of age and older, beginning with the 2016–2017 flu season.[23]
A 2009 meta-analysis assessed data from 64 clinical trials of influenza vaccines with the squalene-containing adjuvant MF59 and compared them to the effects of vaccines with no adjuvant. The analysis reported that the adjuvated vaccines were associated with slightly lower risks of chronic diseases, but that neither type of vaccines altered the rate of autoimmune diseases; the authors concluded that their data "supports the good safety profile associated with MF59-adjuvated influenza vaccines and suggests there may be a clinical benefit over non-MF59-containing vaccines".[24]
Safety
Toxicology studies indicate that in the concentrations used in cosmetics, squalene has low acute toxicity, and is not a significant contact allergen or irritant.[25][26]
The World Health Organization and the US Department of Defense have both published extensive reports that emphasise that squalene is naturally occurring, even in oils of human fingerprints.[11][27] The WHO goes further to explain that squalene has been present in over 22 million flu vaccines given to patients in Europe since 1997 without significant vaccine-related adverse events.[11]
Controversies
Attempts to link squalene to Gulf War Syndrome have been debunked.[28][29][30][31]
References
- ↑ CID 1105 from PubChem
- ↑ Merck Index, 11th Edition, 8727
- ↑ Pabst, Florian; Blochowicz, Thomas (December 2022). "On the intensity of light scattered by molecular liquids - Comparison of experiment and quantum chemical calculations" (in en). The Journal of Chemical Physics 157 (24): 244501. doi:10.1063/5.0133511. PMID 36586992. Bibcode: 2022JChPh.157x4501P.
- ↑ Ernst, Josef; Sheldrick, William S.; Fuhrhop, Juergen H. (December 1976). "Crystal structure of squalene" (in de). Angewandte Chemie 88 (24): 851. doi:10.1002/ange.19760882414.
- ↑ Ronco, Alvaro L.; De Stéfani, Eduardo (20 December 2013). "Squalene: a multi-task link in the crossroads of cancer and aging". Functional Foods in Health and Disease 3 (12): 462–476. doi:10.31989/ffhd.v3i12.30. ISSN 2160-3855. https://ffhdj.com/index.php/ffhd/article/view/30.
- ↑ Pappas, A (1 April 2009). "Epidermal surface lipids". Dermato-Endocrinology (Taylor & Francis) 1 (2): 72–76. doi:10.4161/derm.1.2.7811. PMID 20224687.
- ↑ Micera, Marco; Botto, Alfonso; Geddo, Federica; Antoniotti, Susanna; Bertea, Cinzia Margherita; Levi, Renzo; Gallo, Maria Pia; Querio, Giulia (2 August 2020). "Squalene: More than a Step toward Sterols". Antioxidants 9 (8): 688. doi:10.3390/antiox9080688. PMID 32748847.
- ↑ Cerqueira, Nuno M. F. S. A.; Oliveira, Eduardo F.; Gesto, Diana S.; Santos-Martins, Diogo; Moreira, Cátia; Moorthy, Hari N.; Ramos, Maria J.; Fernandes, P. A. (4 October 2016). "Cholesterol Biosynthesis: A Mechanistic Overview". Biochemistry 55 (39): 5483–5506. doi:10.1021/acs.biochem.6b00342. PMID 27604037.
- ↑ ZANDEE, DI (27 June 1964). "Absence of Sterol Synthesis in some Arthropods". Nature 202 (4939): 1335–6. doi:10.1038/2021335a0. PMID 14210972. Bibcode: 1964Natur.202.1335Z.
- ↑ Abe, Ikuro (2007). "Enzymatic synthesis of cyclic triterpenes". Natural Product Reports 24 (6): 1311–1331. doi:10.1039/b616857b. PMID 18033581.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Squalene-based adjuvants in vaccines". Global Advisory Committee on Vaccine Safety (World Health Organization). 21 July 2006. https://www.who.int/vaccine_safety/committee/topics/adjuvants/squalene/questions_and_answers/en/.
- ↑ Bloch, Konrad E. (1983). "Sterol, Structure and Membrane Function". Critical Reviews in Biochemistry and Molecular Biology 14 (1): 47–92. doi:10.3109/10409238309102790. PMID 6340956.
- ↑ Cerqueira, Nuno M. F. S. A.; Oliveira, Eduardo F.; Gesto, Diana S.; Santos-Martins, Diogo; Moreira, Cátia; Moorthy, Hari N.; Ramos, Maria J.; Fernandes, P. A. (4 October 2016). "Cholesterol Biosynthesis: A Mechanistic Overview". Biochemistry 55 (39): 5483–5506. doi:10.1021/acs.biochem.6b00342. PMID 27604037.
- ↑ Rohmer, M.; Bouvier-Nave, P.; Ourisson, G. (1 May 1984). "Distribution of Hopanoid Triterpenes in Prokaryotes". Microbiology 130 (5): 1137–1150. doi:10.1099/00221287-130-5-1137.
- ↑ Santana-Molina, Carlos; Rivas-Marin, Elena; Rojas, Ana M; Devos, Damien P (1 July 2020). "Origin and Evolution of Polycyclic Triterpene Synthesis". Molecular Biology and Evolution 37 (7): 1925–1941. doi:10.1093/molbev/msaa054. PMID 32125435.
- ↑ Eggersdorfer, Manfred (15 June 2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_205.
- ↑ Bowman, Emma (10 October 2020). "A Coronavirus Vaccine Could Kill Half A Million Sharks, Conservationists Warn". https://www.npr.org/sections/coronavirus-live-updates/2020/10/10/922398246/a-coronavirus-vaccine-could-kill-half-a-million-sharks-conservationists-warn.
- ↑ Mendes, Adélia; Azevedo-Silva, João; Fernandes, João C. (2022-02-22). "From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants". Pharmaceuticals 15 (3): 265. doi:10.3390/ph15030265. ISSN 1424-8247. PMID 35337064.
- ↑ Spanova, Miroslava; Daum, Günther (17 August 2011). "Squalene - biochemistry, molecular biology, process biotechnology, and applications". European Journal of Lipid Science and Technology 113 (11): 1299–1320. doi:10.1002/ejlt.201100203.
- ↑ Pan, Jian-Jung; Solbiati, Jose O.; Ramamoorthy, Gurusankar; Hillerich, Brandan S.; Seidel, Ronald D.; Cronan, John E.; Almo, Steven C.; Poulter, C. Dale (20 April 2015). "Biosynthesis of Squalene from Farnesyl Diphosphate in Bacteria: Three Steps Catalysed by Three Enzymes". ACS Central Science 1 (2): 77–82. doi:10.1021/acscentsci.5b00115. PMID 26258173.
- ↑ Mosca, Frank J.; Tritto, Elaine; Muzzi, Alessandro; Monaci, Ernesto; Bagnoli, Franco; Iavarone, Claudia; O'Hagan, Derek; Rappuoli, Rino et al. (29 July 2008). "Molecular and cellular signatures of human vaccine adjuvants". Proceedings of the National Academy of Sciences 105 (30): 10501–10506. doi:10.1073/pnas.0804699105. PMID 18650390. Bibcode: 2008PNAS..10510501M.
- ↑ Kalvodova, Lucie (12 March 2010). "Squalene-based oil-in-water emulsion adjuvants perturb metabolism of neutral lipids and enhance lipid droplet formation". Biochemical and Biophysical Research Communications 393 (3): 350–355. doi:10.1016/j.bbrc.2009.12.062. PMID 20018176.
- ↑ "FLUAD, Flu Vaccine With Adjuvant". Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. 14 December 2017. https://www.cdc.gov/flu/protect/vaccine/adjuvant.htm.
- ↑ Pellegrini, Michele; Nicolay, Uwe; Lindert, Kelly; Groth, Nicola; Della Cioppa, Giovanni (16 November 2009). "MF59-adjuvated versus non-adjuvated influenza vaccines: Integrated analysis from a large safety database". Vaccine 27 (49): 6959–6965. doi:10.1016/j.vaccine.2009.08.101. PMID 19751689.
- ↑ "Final Report on the Safety Assessment of Squalane and Squalene". International Journal of Toxicology 1 (2): 37–56. 1982. doi:10.3109/10915818209013146. http://www.beauty-review.nl/wp-content/uploads/2014/08/Final-Report-on-the-Safety-Assessment-of-Squalane-and-Squalene.pdf.
- ↑ Huang, Zih-Rou; Lin, Yin-Ku; Fang, Jia-You (16 November 2009). "Biological and Pharmacological Activities of Squalene and Related Compounds: Potential Uses in Cosmetic Dermatology". Molecules 14 (1): 540–554. doi:10.3390/molecules14010540. PMID 19169201. PMC 6253993. http://skinbio.com/squalene-skin.pdf.
- ↑ Asano, Keiji G.; Bayne, Charles K.; Horsman, Katie M.; Buchanan, Michelle V. (17 January 2002). "Chemical Composition of Fingerprints for Gender Determination". Journal of Forensic Sciences 47 (4): 805–807. doi:10.1520/JFS15460J. PMID 12136987.
- ↑ Sox, Harold C.; Fulco, Carolyn; Liverman, Catharyn T. (2000). Gulf War and Health. National Academies Press. p. 311. ISBN 978-0-30907-178-9. https://archive.org/details/gulfwarhealth00fulc.
- ↑ Del Giudice, Giuseppe; Fragapane, Elena; Bugarini, Roberto; Hora, Maninder; Henriksson, Thomas; Palla, Emanuela; O'Hagan, Derek; Donnelly, John et al. (7 September 2006). "Vaccines with the MF59 Adjuvant Do Not Stimulate Antibody Responses against Squalene". Clinical and Vaccine Immunology 13 (9): 1010–1013. doi:10.1128/CVI.00191-06. PMID 16960112.
- ↑ "Gulf War illnesses: Questions About the Presence of Squalene Antibodies in Veterans Can Be Resolved". U.S. Government Accountability Office. March 1999. https://www.gao.gov/archive/1999/ns99005.pdf.
- ↑ Jess Henig (18 Oct 2009). "FactCheck: Swine Flu Vaccine Fears Greatly Exaggerated". Newsweek. https://www.newsweek.com/factcheck-swine-flu-vaccine-fears-greatly-exaggerated-81069.
External links
 |