Chemistry:Aminoacetone
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Aminopropan-2-one[1] | |
| Other names
Aminoacetone[1]
alpha-Aminoacetone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.095 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
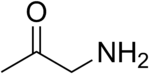
Aminoacetone is the organic compound with the formula CH3C(O)CH2NH2. Although stable in the gaseous form, once condensed it reacts with itself. The protonated derivative forms isolable salts, e.g. aminoacetone hydrochloride ([CH3C(O)CH2NH3]Cl)). The semicarbazone of the hydrochloride is another bench-stable precursor.[2] Aminoacetone is a metabolite that is implicated in the biosynthesis of methylglyoxal.[3]
See also
- Propanolamines
- Aminoaldehydes and aminoketones
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 63. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ John D. Hepworth (1965). "Aminoacetone Semicarbazone Hydrochloride". Organic Syntheses 45: 1. doi:10.15227/orgsyn.045.0001.
- ↑ Bechara, Etelvino J.H.; Dutra, Fernando; Cardoso, Vanessa E.S.; Sartori, Adriano; Olympio, Kelly P.K.; Penatti, Carlos A.A.; Adhikari, Avishek; Assunção, Nilson A. (2007). "The dual face of endogenous α-aminoketones: Pro-oxidizing metabolic weapons". Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 146 (1–2): 88–110. doi:10.1016/j.cbpc.2006.07.004. PMID 16920403.
 |

