Chemistry:Anziaic acid
| |
| Names | |
|---|---|
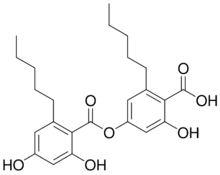
| IUPAC name
4-(2,4-Dihydroxy-6-pentylbenzoyl)oxy-2-hydroxy-6-pentylbenzoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C24H30O7 | |
| Molar mass | 430.5 g/mol |
| Melting point | 122 °C (252 °F; 395 K) dec |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Anziaic acid is a depside found in lichens. It gives a red reaction in the C test. The two phenolic rings have a pentyl side chain. It is an ester dimer of olivetolic acid.[1]
Anziaic acid works as an antibacterial compound by inhibiting topoisomerase.[2][3]
Production
Anziaic acid has been artificially produced from olivetolic acid by benzylation of the O-phenol positions, and then condensing with trifluoroacetic anhydride.[1]
Properties
Anziaic acid is colourless. It can be dissolved in ethanol, ethanol-water mixture, or cyclohexane-benzene mixture.[1]
Related
Perlatolic acid, dihydropicrolichenic acid, 2'-O-methylanziaic acid, 2-O-methylperlatolic acid, 2'-O-methylperlatolic and planaic acid are derivatives of anziaic acid, where a methyl group replaces a hydrogen in some of the hydroxy positions on the rings.[1]
Occurrence
Anziaic acid is found in Parmeliaceae including Hypotrachyna,[2] Stereocaulon,[4] and Cetrelia,[5]
References
- ↑ 1.0 1.1 1.2 1.3 Elix, J. A. (1974). "Synthesis of para-olivetol depsides". Australian Journal of Chemistry 27 (8): 1767. doi:10.1071/CH9741767.
- ↑ 2.0 2.1 Cheng, Bokun; Cao, Shugeng; Vasquez, Victor; Annamalai, Thirunavukkarasu; Tamayo-Castillo, Giselle; Clardy, Jon; Tse-Dinh, Yuk-Ching (8 April 2013). "Identification of Anziaic Acid, a Lichen Depside from Hypotrachyna sp., as a New Topoisomerase Poison Inhibitor". PLOS ONE 8 (4): e60770. doi:10.1371/journal.pone.0060770. PMID 23593306. Bibcode: 2013PLoSO...860770C.
- ↑ Kekuda, T.R Prashith; Lavanya, D .; Pooja, Rao (March 2019). "Lichens as promising resources of enzyme inhibitors: A review". Journal of Drug Delivery and Therapeutics 9 (2S). doi:10.22270/jddt.v9i2-s.2546.
- ↑ Ramaut, J. L.; Serusiaux, E.; Brouers, M.; Corvisier, M. (1978). "Lichen Acids of the Stereocaulon ramulosum Group in Central East Africa". The Bryologist 81 (3): 415. doi:10.2307/3242244.
- ↑ Mark, Kristiina; Randlane, Tiina; Thor, Göran; Hur, Jae-Seoun; Obermayer, Walter; Saag, Andres (2019). "Lichen chemistry is concordant with multilocus gene genealogy in the genus Cetrelia (Parmeliaceae, Ascomycota)". Fungal Biology 123 (2): 125–139. doi:10.1016/j.funbio.2018.11.013. PMID 30709518.
 |

