Chemistry:Armstrong's acid
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Naphthalene-1,5-disulfonic acid | |||
| Other names
Armstrong's acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
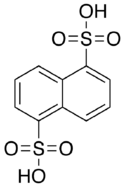
| C10H8S2O6 | |||
| Molar mass | 288.299 g/mol | ||
| Appearance | colorless solid | ||
| Hazards | |||
| Main hazards | corrosive | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Armstrong's acid (naphthalene-1,5-disulfonic acid) is a fluorescent organic compound with the formula C10H6(SO3H)2. It is one of several isomers of naphthalenedisulfonic acid. It a colorless solid, typically obtained as the tetrahydrate.[1] Like other sulfonic acids, it is a strong acid. It is named for British chemist Henry Edward Armstrong.[2]
Production and use
It is prepared by disulfonation of naphthalene with oleum:
- C10H8 + 2 SO3 → C10H6(SO3H)2
Further sulfonation gives The 1,3,5-trisulfonic acid derivative.[1]
Reactions and uses
Fusion of Armstrong's acid in NaOH gives the disodium salt of 1,5-dihydroxynaphthalene, which can be acidified to give the diol. The intermediate in this hydrolysis, 1-hydroxynaphthalene-5-sulfonic acid, is also useful. Nitration gives nitrodisulfonic acids, which are precursors to amino derivatives.
The disodium salt is sometimes used as a divalent counterion for forming salts of basic drug compounds, as an alternative to the related mesylate or tosylate salts. When used in this way such a salt is called a naphthalenedisulfonate salt, as seen with the most common salt form of the stimulant drug CFT. The disodium salt is also used as an electrolyte in certain kinds of chromatography.[3]
References
- ↑ 1.0 1.1 Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_009.
- ↑ Senning, Alexander (2007). Elsevier's dictionary of chemoetymology. Elsevier. pp. 30. ISBN 978-0-444-52239-9. https://books.google.com/books?id=Fl4sdCYrq3cC.
- ↑ Shigeru Terabe "Electrokinetic chromatography: An interface between electrophoresis and chromatography" TrAC Trends in Analytical Chemistry 1989, Volume 8, pp. 129–134. doi:10.1016/0165-9936(89)85022-8
 |