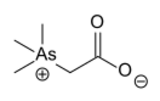
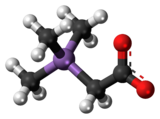
Chemistry:Arsenobetaine
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Trimethylarsaniumyl)acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| 3933180 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | Arsenobetaine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C5H11AsO2 | |
| Molar mass | 177.99/ g.mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H331, H410 | |
| P261, P264, P270, P271, P273, P301+310, P304+340, P311, P321, P330, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Arsenobetaine is an organoarsenic compound that is the main source of arsenic found in fish.[1][2][3][4] It is the arsenic analog of trimethylglycine, commonly known as betaine. The biochemistry and its biosynthesis are similar to those of choline and betaine.
Arsenobetaine is a common substance in marine biological systems and unlike many other organoarsenic compounds, such as trimethylarsine, it is relatively non-toxic.[5][6] The compound may play a similar role as urea does for nitrogen, as a non-toxic waste compound made in the bodies of animals to dispose of the relevant element.
It has been known since 1920 that marine fish contain organoarsenic compounds, but it was not until 1977 that the chemical structure of the most predominant compound arsenobetaine was determined.[7]
Safety
Whereas arsenous acid (As(OH)3) has an LD50 (mice) of 34.5 mg/kg (mice), the LD50 for the arsenobetaine exceeds 10 g/kg.[8]
References
- ↑ Maher, B. (2005). "Foreword: Research Front — Arsenic Biogeochemistry". Environmental Chemistry 2 (3): 139–140. doi:10.1071/EN05063.
- ↑ Francesconi, K. A. (2005). "Current Perspectives in Arsenic Environmental and Biological Research". Environmental Chemistry 2 (3): 141–145. doi:10.1071/EN05042.
- ↑ Adair, B. M.; Waters, S. B.; Devesa, V.; Drobna, Z.; Styblo, M.; Thomas, D. J. (2005). "Commonalities in Metabolism of Arsenicals". Environmental Chemistry 2 (3): 161–166. doi:10.1071/EN05054. https://zenodo.org/record/1236044.
- ↑ Ng, J. C. (2005). "Environmental Contamination of Arsenic and its Toxicological Impact on Humans". Environmental Chemistry 2 (3): 146–160. doi:10.1071/EN05062.
- ↑ "Bioaccumulation and biotransformation of arsenic compounds in Hediste diversicolor (Muller 1776) after exposure to spiked sediments". Environmental Science and Pollution Research 21 (9): 5952–5959. 2014. doi:10.1007/s11356-014-2538-z. PMID 24458939. https://link.springer.com/article/10.1007/s11356-014-2538-z.
- ↑ Bhattacharya, P.; Welch, A. H.; Stollenwerk, K. G.; McLaughlin, M. J.; Bundschuh, J.; Panaullah, G. (2007). "Arsenic in the Environment: Biology and Chemistry". Science of the Total Environment 379 (2–3): 109–120. doi:10.1016/j.scitotenv.2007.02.037. PMID 17434206. Bibcode: 2007ScTEn.379..109B.
- ↑ Edmonds, J. S.; Francesconi, K. A.; Cannon, J. R.; Raston, C. L.; Skelton, B. W.; White, A. H. (1977). "Isolation, Crystal Structure and Synthesis of Arsenobetaine, the Arsenical Constituent of the Western Rock Lobster Panulirus longipes cygnus George". Tetrahedron Letters 18 (18): 1543–1546. doi:10.1016/S0040-4039(01)93098-9.
- ↑ Cullen, William R.; Reimer, Kenneth J. (1989). "Arsenic speciation in the environment". Chemical Reviews 89 (4): 713–764. doi:10.1021/cr00094a002.
Further reading
- Craig, P. J. (2003). Organometallic Compounds in the Environment (2nd ed.). Chichester: John Wiley and Sons. pp. 415. ISBN 978-0-471-89993-8. https://books.google.com/books?id=zdjwlDvtzUYC.
 |
