Chemistry:Trimethylglycine
| |
| |
| Names | |
|---|---|
| IUPAC name
(Trimethylammonio)acetate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3537113 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 26434 | |
| KEGG | |
| MeSH | Betaine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H11NO2 | |
| Molar mass | 117.146 |
| Appearance | White solid |
| Melting point | 180 °C (356 °F; 453 K)[1] (decomposes) |
| Soluble | |
| Solubility | Methanol |
| Acidity (pKa) | 1.84 |
| Pharmacology | |
| 1=ATC code }} | A16AA06 (WHO) |
| License data | |
| By mouth | |
| Legal status | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
| Related compounds | |
Related amino acids
|
Glycine Methylglycine Dimethylglycine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
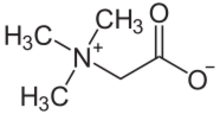
Trimethylglycine is an amino acid derivative that occurs in plants. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because, in the 19th century, it was discovered in sugar beets (Beta vulgaris subsp. vulgaris).[6]
Medical uses
Betaine, sold under the brand name Cystadane among others, is indicated for the adjunctive treatment of homocystinuria, involving deficiencies or defects in cystathionine beta-synthase (CBS), 5,10-methylene-tetrahydrofolate reductase (MTHFR), or cobalamin cofactor metabolism (cbl).[3][4][5]
The most common side effect is elevated levels of methionine in the blood.[4]
Structure and reactions
Trimethylglycine is an N-methylated amino acid. It is a zwitterion as the molecule contains both a quaternary ammonium group and a carboxyl group. The carboxyl group will be partially protonated in aqueous solution below pH 4, that is, approximately below pH equal to (pKa + 2).
- (CH
3)
3N+
CH
2CO−
2 (aq) + H+
⇌ (CH
3)
3N+
CH
2CO
2H (aq)
Demethylation of trimethylglycine gives dimethylglycine.
Production and biochemical processes
Processing sucrose from sugar beets yields glycine betaine as a byproduct. The economic value of the trimethylglycine rivals that of the sugar content in sugar beets.[7]
Biosynthesis
In most organisms, glycine betaine is biosynthesized by oxidation of choline in two steps. The intermediate, betaine aldehyde, is generated by the action of the enzyme mitochondrial choline oxidase (choline dehydrogenase, EC 1.1.99.1). Betaine aldehyde is further oxidised in the mitochondria in mice to betaine by the enzyme betaine-aldehyde dehydrogenase (EC 1.2.1.8).[8][9] In humans betaine aldehyde activity is performed by a nonspecific cystosolic aldehyde dehydrogenase enzyme (EC 1.2.1.3) [10]
Biological function
Trimethylglycine is an organic osmolyte. Sugar beet was cultivated from sea beet, which requires osmolytes in order to survive in the salty soils of coastal areas. Trimethylglycine also occurs in high concentrations (~10 mM) in many marine invertebrates, such as crustaceans and molluscs. It serves as a potent appetitive attractant to generalist carnivores such as the predatory sea slug Pleurobranchaea californica.[11]
Trimethylglycine is an important cofactor in methylation, a process that occurs in every mammalian cell donating methyl groups (–CH3) for other processes in the body. These processes include the synthesis of neurotransmitters such as dopamine and serotonin. Methylation is also required for the biosynthesis of melatonin and the electron transport chain constituent coenzyme Q10, as well as the methylation of DNA for epigenetics.
The major step in the methylation cycle is the remethylation of homocysteine, a compound which is naturally generated during demethylation of the essential amino acid methionine. Despite its natural formation, homocysteine has been linked to inflammation, depression, specific forms of dementia, and various types of vascular disease. The remethylation process that detoxifies homocysteine and converts it back to methionine can occur via either of two pathways. The pathway present in virtually all cells involves the enzyme methionine synthase (MS), which requires vitamin B12 as a cofactor, and also depends indirectly on folate and other B vitamins. The second pathway (restricted to liver and kidney in most mammals) involves betaine-homocysteine methyltransferase (BHMT) and requires trimethylglycine as a cofactor. During normal physiological conditions, the two pathways contribute equally to removal of homocysteine in the body.[12] Further degradation of betaine, via the enzyme dimethylglycine dehydrogenase produces folate, thus contributing back to methionine synthase. Betaine is thus involved in the synthesis of many biologically important molecules, and may be even more important in situations where the major pathway for the regeneration of methionine from homocysteine has been compromised by genetic polymorphisms such as mutations in the MS gene.
Trimethylglycine is produced by some cyanobacteria. Gabbay-Azaria et al 1988 uses 13C nuclear magnetic resonance to detect trimethylglycines produced by halophilic cyanobacteria. They find it is providing partial protection for their enzymes, against inhibition by NaCl and KCl.[13]
Agriculture and aquaculture
Factory farms supplement fodder with trimethylglycine and lysine to increase livestock's muscle mass (and, therefore, "carcass yield", the amount of usable meat).
Salmon farms apply trimethylglycine to relieve the osmotic pressure on the fishes' cells when workers transfer the fish from freshwater to saltwater.[7][14]
Trimethylglycine supplementation decreases the amount of adipose tissue in pigs; however, research in human subjects has shown no effect on body weight, body composition, or resting energy expenditure.[15]
Nutrition
Nutritionally, betaine is not needed when sufficient dietary choline is present for synthesis.[16] When insufficient betaine is available, elevated homocysteine levels and decreased SAM levels in blood occur. Supplementation of betaine in this situation would resolve these blood marker issues, but not compensate for other functions of choline.[17]
| Food | Betaine (mg/100 g) |
|---|---|
| Quinoa | 630 |
| Wheat germ | 410 |
| Lamb's quarters | 330 |
| Wheat bran | 320 |
| Canned Beetroot | 260 |
| Dark Rye flour | 150 |
| Spinach | 110-130 |
Dietary supplement
Although trimethylglycine supplementation decreases the amount of adipose tissue in pigs, research on human subjects has shown no effect on body weight, body composition, or resting energy expenditure when used in conjunction with a low calorie diet.[15] The US Food and Drug Administration (FDA) approved betaine trimethylglycine (also known by the brand name Cystadane) for the treatment of homocystinuria, a disease caused by abnormally high homocysteine levels at birth.[19] Trimethylglycine is also used as the hydrochloride salt (marketed as betaine hydrochloride or betaine HCl). Betaine hydrochloride was sold over-the-counter (OTC) as a gastric aid in the United States. US Code of Federal Regulations, Title 21, Section 310.540, which became effective in November 1993, banned the marketing of betaine hydrochloride as a digestive aid due to insufficient evidence to classify it as "generally recognized as safe and effective" for that specified use.[20]
Side effects
Trimethylglycine supplementation may cause diarrhea, bloating, cramps, dyspepsia, nausea or vomiting.[21] Although rare, it can also causes excessive increases in serum methionine concentrations in the brain, which may lead to cerebral edema, a life-threatening condition.[21]
Trimethylglycine supplementation lowers homocysteine but also raises LDL-cholesterol in obese individuals and renal patients.[22]
Other uses
Polymerase chain reaction
Trimethylglycine can act as an adjuvant of the polymerase chain reaction (PCR) process, and other DNA polymerase-based assays such as DNA sequencing. By an unknown mechanism, it aids in the prevention of secondary structures in the DNA molecules, and prevents problems associated with the amplification and sequencing of GC-rich regions. Trimethylglycine makes guanosine and cytidine (strong binders) behave with thermodynamics similar to those of thymidine and adenosine (weak binders). It has been determined under experiment that it is best used at a final concentration of 1 M.[23]
References
- ↑ Acheson, R. M.; Bond, G. J. F. (1956). "52. Addition reactions of heterocyclic compounds. Part II. Phenanthridine and methyl acetylenedicarboxylate in methanol". J. Chem. Soc. 1956: 246. doi:10.1039/JR9560000246.
- ↑ "Notice of Amendment: Betaine removed from the Prescription Drug List (PDL)". 6 January 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/amendment-betaine-removed.html.
- ↑ 3.0 3.1 "Cystadane- betaine powder, for solution". 3 October 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=31a684d0-cd98-4e46-b43a-ccbd17c41559.
- ↑ 4.0 4.1 4.2 "Cystadane EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/cystadane. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 5.0 5.1 "Amversio EPAR". 21 February 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/amversio. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Schiweck, Hubert; Clarke, Margaret; Pollach, Günter. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_345.pub2.
- ↑ 7.0 7.1 Mäkelä, P. (2004). "Agro-industrial uses of glycinebetaine". Sugar Tech. 6 (4): 207–212. doi:10.1007/BF02942500.
- ↑ Kempf, B.; Bremer, E. (1998). "Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments". Arch. Microbiol. 170 (5): 319–330. doi:10.1007/s002030050649. PMID 9818351.
- ↑ "BRENDA – Information on EC 1.2.1.8 – betaine-aldehyde dehydrogenase". http://www.brenda-enzymes.org/enzyme.php?ecno=1.2.1.8.
- ↑ Chern, M. K.; Pietruszko, R. (1999). "Evidence for mitochondrial localization of betaine aldehyde dehydrogenase in rat liver: purification, characterization, and comparison with human cytoplasmic E3 isoenzyme". Biochemistry and Cell Biology 77 (3): 179–187. doi:10.1139/o99-030. PMID 10505788.
- ↑ Gillette, R.; Huang, R. C.; Hatcher, N.; Moroz, L. L. (March 2000). "Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain". Proc. Natl. Acad. Sci. USA 97 (7): 3585–3590. doi:10.1073/pnas.97.7.3585. PMID 10737805. Bibcode: 2000PNAS...97.3585G.
- ↑ Finkelstein, J. D. (24 March 1998). "The metabolism of homocysteine: pathways and regulation" (in en). European Journal of Pediatrics 157 (S2): S40–S44. doi:10.1007/pl00014300. ISSN 0340-6199. PMID 9587024.
- ↑ Rhodes, D.; Hanson, A. D. (1993). "Quaternary Ammonium and Tertiary Sulfonium Compounds in Higher Plants". Annual Review of Plant Physiology and Plant Molecular Biology (Annual Reviews) 44 (1): 357–384. doi:10.1146/annurev.pp.44.060193.002041. ISSN 1040-2519.
- ↑ Xue, M.; Xie, S.; Cui, Y. (2004). "Effect of a feeding stimulant on feeding adaptation of gibel carp Carassius auratus gibelio (Bloch), fed diets with replacement of fish meal by meat and bone meal". Aquaculture Research 35 (5): 473–482. doi:10.1111/j.1365-2109.2004.01041.x.
- ↑ 15.0 15.1 Schwab, U. et al. (November 2002). "Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects". Am. J. Clin. Nutr. 76 (5): 961–967. doi:10.1093/ajcn/76.5.961. PMID 12399266.
- ↑ Handbook of vitamins (4th ed.). Taylor & Francis. 2007. pp. 459–477. ISBN 9780849340222. https://archive.org/details/handbookvitamins00jzem.
- ↑ "Dietary reference values for choline". EFSA Journal 14 (8). 2016. doi:10.2903/j.efsa.2016.4484.
- ↑ "USDA Database for the Choline Content of Common Foods, Release 2 (2008)". 1 November 2019. https://data.nal.usda.gov/dataset/usda-database-choline-content-common-foods-release-2-2008.
- ↑ Holm, P. I. et al. (February 2005). "Betaine and folate status as cooperative determinants of plasma homocysteine in humans". Arterioscler. Thromb. Vasc. Biol. 25 (2): 379–385. doi:10.1161/01.ATV.0000151283.33976.e6. PMID 15550695.
- ↑ "CFR - Code of Federal Regulations Title 21". https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=310.545&SearchTerm=betaine%20hydrochloride.
- ↑ 21.0 21.1 "Betaine", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases), 2012, PMID 31644082, http://www.ncbi.nlm.nih.gov/books/NBK548774/, retrieved 2023-07-14
- ↑ "Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans". PLOS Med. 2 (5): e135. 2005. doi:10.1371/journal.pmed.0020135. PMID 15916468.
- ↑ Henke, W.; Herdel, K.; Jung, K.; Schnorr, D.; Loening, S. A. (October 1997). "Betaine improves the PCR amplification of GC-rich DNA sequences.". Nucleic Acids Res. 25 (19): 3957–3958. doi:10.1093/nar/25.19.3957. PMID 9380524. PMC 146979. http://www.pubmed.com/9380524.
External links
- USDA Database for the Choline Content of Common Foods – including the data on choline metabolites, such as betaine, in 434 food items.
 |
