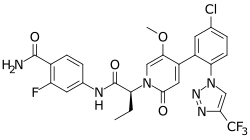
Chemistry:Asundexian
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H21ClF4N6O4 |
| Molar mass | 592.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Asundexian is a Factor XIa inhibitor developed by Bayer to prevent stroke.[1]
Clinical trial(s)
Asundexian efficacy and safety in patients have been evaluated in two clinical trial programs: phase IIb PACIFIC and phase III OCEANIC.[2] In the phase IIb PACIFIC clinical trial programs, asundexian consistently showed no difference in bleeding rate compared with placebo[3][4] and reduced the risk of bleeding compared with apixaban.[5] All three trials in the programs were not powered to show efficacy of asundexian.[2] However, a phase III trial, OCEANIC-AF, was stopped due to a "lack of efficacy." The remaining active study OCEANIC-STROKE was recommended to continue as planned. Bayer is reevaluating the design of the OCEANIC-AFINA based on the findings of the OCEANIC trial.[6]
References
- ↑ Heitmeier, Stefan; Visser, Mayken; Tersteegen, Adrian; Dietze-Torres, Julia; Glunz, Julia; Gerdes, Christoph; Laux, Volker; Stampfuss, Jan et al. (June 2022). "Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa". Journal of Thrombosis and Haemostasis 20 (6): 1400–1411. doi:10.1111/jth.15700. PMID 35289054.
- ↑ 2.0 2.1 "Bayer initiates landmark Phase III study program to investigate oral FXIa inhibitor asundexian". 2022-08-28. https://www.bayer.com/media/en-us/bayer-initiates-landmark-phase-iii-study-program-to-investigate-oral-fxia-inhibitor-asundexian/.
- ↑ "AntiCoagulation via Inhibition of FXIa by the Oral Compound BAY 2433334 – Non-cardioembolic Stroke". https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2022/08/27/04/10/http%3a%2f%2fwww.acc.org%2fLatest-in-Cardiology%2fClinical-Trials%2f2022%2f08%2f27%2f04%2f10%2fPACIFIC-STROKE.
- ↑ Rao, Sunil V.; Kirsch, Bodo; Bhatt, Deepak L.; Budaj, Andrzej; Coppolecchia, Rosa; Eikelboom, John; James, Stefan K.; Jones, W. Schuyler et al. (2022-10-18). "A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, Dose-Finding Trial of the Oral Factor XIa Inhibitor Asundexian to Prevent Adverse Cardiovascular Outcomes After Acute Myocardial Infarction" (in en). Circulation 146 (16): 1196–1206. doi:10.1161/CIRCULATIONAHA.122.061612. ISSN 0009-7322. PMID 36030390.
- ↑ "The Next Wave of Anticoagulation: Results of PACIFIC-AF and the Future Role of Factor XIa Inhibition in Atrial Fibrillation". https://www.acc.org/Latest-in-Cardiology/Articles/2022/06/17/11/54/http%3a%2f%2fwww.acc.org%2fLatest-in-Cardiology%2fArticles%2f2022%2f06%2f17%2f11%2f54%2fThe-Next-Wave-of-Anticoagulation.
- ↑ "OCEANIC-AF study stopped early due to lack of efficacy". 2023-11-19. https://www.bayer.com/media/en-us/oceanic-af-study-stopped-early-due-to-lack-of-efficacy/.
 |

