Chemistry:Aszonalenin
From HandWiki
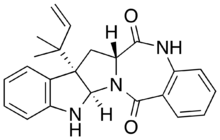
| |
| Names | |
|---|---|
| IUPAC name
(2R,10S,12R)-10-(2-methylbut-3-en-2-yl)-1,3,14-triazapentacyclo[10.9.0.02,10.04,9.015,20]henicosa-4,6,8,15,17,19-hexaene-13,21-dione[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H23N3O2 | |
| Molar mass | 373.456 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aszonalenin is an alkaloid which is produced by Neosartorya and Aspergillus species.[2][3] Aszonalenin is a neurotoxin.[4]
References
- ↑ "Aszonalenin" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/42636439#section=Names-and-Identifiers.
- ↑ Whittall, John; Sutton, Peter W. (16 April 2012) (in en). Practical Methods for Biocatalysis and Biotransformations 2. John Wiley & Sons. p. 254. ISBN 978-1-119-94341-9.
- ↑ Grundon, M. F. (in en). Alkaloids. Royal Society of Chemistry. p. 214. ISBN 978-0-85186-367-2.
- ↑ Lubertozzi, David M. (2009). "Developing Aspergillus as a Host for Heterologous Expression" (in en). Biotechnology Advances (University of California, Berkeley) 27 (1): 53–75. doi:10.1016/j.biotechadv.2008.09.001. PMID 18840517.
Further reading
- Ruchti, Jonathan; Carreira, Erick M. (2014). "Ir-Catalyzed Reverse Prenylation of 3-Substituted Indoles: Total Synthesis of (+)-Aszonalenin and (–)-Brevicompanine B". J. Am. Chem. Soc. 136 (48): 16756–16759. doi:10.1021/ja509893s. PMID 25365411.
- Kimura, Yasuo; Hamasaki, Takashi; Nakajima, Hiromitsu; Isogai, Akira (1982). "Structure of aszonalenin, a new metabolite of Aspergillus zonatus" (in en). Tetrahedron Letters 23 (2): 225–228. doi:10.1016/S0040-4039(00)86791-X. ISSN 0040-4039.
 |
