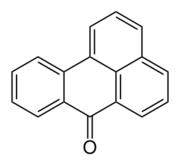
Chemistry:Benzanthrone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
7H-Benzo[de]anthracen-7-one | |
| Other names
Benzanthrenone
1,9-Benzanthrone MS-Benzanthrone Mesobenzanthrone Naphtanthrone 7H-Benz(de)anthracene-7-one 7-Oxobenz(de)anthracene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2811 |
| |
| |
| Properties | |
| C17H10O | |
| Molar mass | 230.266 g·mol−1 |
| Appearance | Light yellow to brown-green solid |
| Melting point | 170 °C (338 °F; 443 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzanthrone (BZA) is a polycyclic aromatic hydrocarbon. It is a yellow solid.[1] Its derivatives are used as a dyestuff intermediate for anthraquinone-based dyes.[2] Dehydrogenative coupling gives violanthrone. It is prepared by reduction of anthroquinone to anthrone followed by alkylation with a mixture of glycerol and sulfuric acid.
It is a basic substance with fluorescent and luminescent properties. It can be used for photosensitization, and as a charge transport material. It is also used in pyrotechnics industry, mainly as a component of some older formulations of green and yellow colored smokes, often together with Vat Yellow 4; its US military specification is MIL-D-50074D.[3]
Safety
Benzanthrone causes itching and burning sensations on exposed skin, together with erythema, dermatitis, and skin pigmentation.[4]
See also
References
- ↑ Macleod, L. C.; Allen, C. F. H. (1934). "Benzanthrone". Organic Syntheses 14: 4. doi:10.15227/orgsyn.014.0004.
- ↑ Bien, H.-S.; Stawitz, J.; Wunderlich, K. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- ↑ Benzanthrone. National Academies Press (US). 1999. https://www.ncbi.nlm.nih.gov/books/NBK224737/.
- ↑ "Appendix A: Benzanthrone". Toxicity of Military Smokes and Obscurants. 3. 1999. http://www.nap.edu/books/0309065992/html/50.html.
External links
 |
