Chemistry:Bismarck brown Y
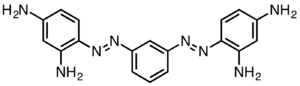
| |
| Names | |
|---|---|
| Other names
Bismarck brown
Manchester brown Phenylene brown Basic Brown 1 C.I. 21000 Vesuvine BA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H18N8·2HCl | |
| Molar mass | 419.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bismarck brown Y also called C.I. 21000[1] and C.I. Basic Brown 1,[1] is a diazo dye with the idealized formula [(H2N)2C6H3N2]2C6H4. The dye is a mixture of closely related compounds. It was one of the earliest azo dyes, being described in 1863 by German chemist Carl Alexander von Martius. It is used in histology for staining tissues.[2]
Synthesis
The dye is simple to prepare because the diamine serves both as a source of the diazonium cation and as the coupling partner in the azo coupling reaction. The synthesis is thought to start with double diazotization of 1,3-phenylenediamine:
- (H2N)2C6H4 + 2 H+ + 2 HNO2 → [C6H4(N2)2]2+ + 2 H2O
It is assumed that this bis(diazonium) ion subsequently attacks two equivalents of 1,3-phenylenediamine:
- 2 (H2N)2C6H4 + [C6H4(N2)2]2+ → 2 H+ + [(H2N)2C6H3N2]2C6H4
In some cases, toluenediamines are used in addition to the phenylenediamine. Furthermore, the resulting dye is thought to consist of oligomers with three or more diazo groups.[2]
Uses
Bismarck brown Y stains acid mucins to yellow color. It also stains mast cell granules brown.[3] It can be used with live cells. It is also used to stain cartilage in bone specimens, as one of Kasten's Schiff-type reagents in the periodic acid-Schiff stain to stain cellulose, and in Feulgen stain to stain DNA. It was more common in the past; today it is partially replaced by other stains. It has also been used to give soap an amber color in the past.[4]
Bismarck brown Y is a constituent of Papanicolaou stain.[1]
It can also be used as a counterstain for Victoria blue R for staining of acid-fast microorganisms.
References
- ↑ Jump up to: 1.0 1.1 1.2 Lillie, Ralph Dougall (1977). H. J. Conn's Biological stains (9th ed.). Baltimore: Williams & Wilkins. pp. 692p.
- ↑ Jump up to: 2.0 2.1 Booth, Gerald (2000). "Dyes, General Survey". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_073. ISBN 3527306730.
- ↑ Tomov, Nikola; Dimitrov, Nikolay (2017). "Modified Bismarck brown staining for demonstration of soft tissue mast cells". Trakia Journal of Sciences 15 (3): 195–197. doi:10.15547/tjs.2017.03.001. http://tru.uni-sz.bg/tsj/Vol15_N3_2017/2_N.Tomov.pdf.
- ↑ The Chemical Formulary. David Van Nostrand Company Inc.. 1933. p. 86. https://archive.org/details/in.ernet.dli.2015.239029.
 |

