Chemistry:Bleach activator


Bleach activators are compounds that allow a lower washing temperature than would be required otherwise to achieve the full activity of bleaching agents in the wash liquor. Bleaching agents, usually peroxides, are usually sufficiently active only at 60 °C and up. With bleach activators, this activity can be achieved at lower temperatures. Bleach activators are included in some laundry detergent powders (e.g. Tide), some laundry additive powders, and a few[1] laundry additive pods. They are not included in any liquid laundry detergents. Bleach activators react with hydrogen peroxide in aqueous solution to form peroxy acids. Peroxy acids are more active bleaches than hydrogen peroxide at lower temperatures (<60 °C), but are too unstable to be stored in their active form, and hence must be generated in situ.
The most common bleach activators used commercially are tetraacetylethylenediamine (TAED) and sodium nonanoyloxybenzenesulfonate (NOBS). NOBS is the main activator used in the U.S.A. and Japan,[2] TAED is the main activator used in Europe.[3]
Structure and properties
Bleach activators are typically made up of two parts: the peroxy acid precursor and the leaving group; and are modified by altering these parts. The peroxy acid precursor affects the bleaching properties of the peroxy acid: determining the activity, selectivity, hydrophobic/hydrophilic balance and oxidation potential. The leaving group influences the solubility, perhydrolysis rate and storage stability of the activator.[4]
Mechanism of activation
Bleach activation is also known as perhydrolysis. Persalts are inorganic salts that are used as hydrogen peroxide carriers (examples include sodium percarbonate and sodium perborate). Persalts and bleach activators are included together in powder laundry detergents that contain bleach. In the wash, both compounds dissolve in the water. When dissolved in water, the persalt releases hydrogen peroxide (e.g. from sodium percarbonate):
- 2Na2CO3∙3H2O2 → 2Na2CO3 + 3H2O2
In a basic wash solution, hydrogen peroxide loses a proton and is converted to the perhydroxyl anion:
- H2O2 ⇌ H+ + HO2−
The perhydroxyl anion then attacks the activator, forming a peroxy acid:
- HO2− + RC(O)X → X− + RC(O)O2H
The overall reaction of TAED (1) with 2 equivalents of hydrogen peroxide gives diacetylethylenediamine (2) and 2 equivalents of peracetic acid (3):
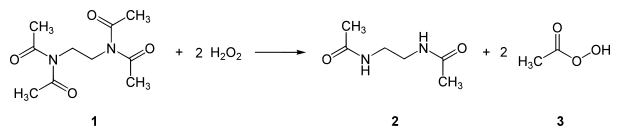
Only the perhydroxyl anion, and not the hydrogen peroxide molecule, reacts with the bleach activator.[5] In aqueous solutions, the hydroxide ion is also present, but owing to the greater nucleophilicity of the perhydroxyl anion, it will react preferentially. Once formed, the peroxy acid can act as a bleach.
Economics
The consumption of bleach activators in 2002 was approximately 105,000 tonnes.[6] Consumption, however, is stagnant or declining due to cost pressures on detergents and the advance of liquid detergent formulations (which contain no bleach and bleach activators). The relatively high cost of conventional bleaching systems restrict their spread in emerging markets, where cold water is used for washing and photobleaching by sunlight is widespread, or the use of sodium hypochlorite solution is common (as in the US).
There remains considerable potential in Europe for more active bleach activators due to the significant potential energy savings achievable by washing at lower temperatures, but their higher activity must not be accompanied by greater damage to textile dyes and fibers. In addition to stain bleaching in laundry, the disinfecting and deodorizing effects of bleach/activator combinations also play an important role. Therefore, they are also used in dishwashing detergents and denture cleaners.[7]
Examples
Typical bleach activators are essentially N- and O-acyl compounds that form peroxyacids upon perhydrolysis (meaning hydrolysis by hydrogen peroxide from the bleach, persalts). For example, TAED produces in the wash liquor bleach-active peroxyacetic acid or from DOBA peroxydodecanoic acid. In all cases, the activator is chemically reacted according to the degree of contamination in the laundry and thus "consumed".
The literature describes a variety of active N-acyl compounds, such as tetraacetyl glycoluril and other acylated saturated nitrogen-containing heterocycles, such as hydantoins, hydrotriazines, diketopiperazines, etc., as well as acylated imides and lactams. A disadvantage of these compounds compared to the standard compound TAED is their usually poorer economic and ecological performance.
In addition to the acylated phenol derivatives NOBS, LOBS and DOBA (negatively charged in the aqueous medium), further bleach-active O-acyl compounds are described, for example tetraacetylxylose or pentaacetylglucose. DOBA, commonly used in Japan, is characterized by good biodegradability and greater effect on a number of microorganisms compared to TAED. Both work together synergistically.[8] Furthermore, nitriles, such as cyanopyridine and cyanamides, cyanomorpholine and in particular cyanomethyl trialkyl/arylammonium salts are known bleach activators (the latter, the so-called nitrile quats,[9][10] are present in aqueous solution as cations).
Nitrile quats are active in bleaching even at temperatures around 20 °C and act via peroxoimino acids that are formed intermediately from peroxo compounds. These decompose to the corresponding quaternary amides, which react with the help of hydrogen peroxide to the corresponding, readily biodegradable betaines.[11] A disadvantage of nitrile quats is the poor biodegradability of the original substances and their often pronounced hygroscopicity, which, however, can be reduced by suitable counterions.
Other new bleaching systems have been developed, especially for washing at lower temperatures and room temperature and for use in liquid detergent formulations:
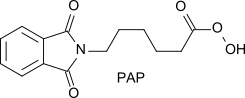
- New and more active peroxyacids, such as phthalimidoperoxyhexanoic acid (PAP)[12]
- Peracid boosters that form highly reactive intermediates with peracids (such as cyclic sulfonimines as precursors of reactive oxaziridines[13]) or sugar-based ketones that form bleach-active dioxiranes with hydrogen peroxide[14]
- Bleach catalysts, which form as stable transition metal complexes (of metals such as manganese, iron, cobalt, etc.) with persalts bleaching-active oxygen species even at temperatures below 30 °C. They exceeds the activity of the standard compound TAED by almost 100 times.[15] Such complexes offer enormous economic (lower detergent volume, less packaging, lower transport costs) and environmental benefits (low washing temperature, low wastewater pollution). Particularly interesting are bleaching catalysts of the second generation, which already form bleaching-active species with atmospheric oxygen, i.e. they can mimic the active sites of natural mono- or dioxygenases. However, in 1994, the launch of a first generation manganese complex[16] ("persil power flop") by Unilever in the UK failed disastrously. As a result, consumers' confidence in bleach catalysts has been shaken on a sustained basis.[17] The only use of bleach catalyst/persalt combinations to date (2017) is used in dishwashing detergents.
References
- ↑ "An ode to oxygen bleach: my favorite laundry additive". https://olgaslaundry.com/2017/12/24/ode-oxygen-bleach-favorite-laundry-additive/.
- ↑ Hirschen, M. (2005). Handbook of Detergents Part C: Analysis. Marcel Dekker. pp. 439–470.
- ↑ Grime, K.; Clauss, A. (1990). "Laundry Bleaches and Activators". Chem. Ind. (647–649).
- ↑ Reinhardt, G.; Borchers, G. (2009). Handbook of Detergents, Part E: Applications. Chapter 16: Application of Bleaching Detergent Formulations: CRC Press. pp. 376–412.
- ↑ Hauthal, H. G.; Schmidt, H.; Scholz, H. J.; Hofmann, J.; Pritzkow, W. (1990). "Studies concerning the mechanism of bleaching activation". Tenside Surf. Det. 27 (3): 7. doi:10.1515/tsd-1990-270314.
- ↑ G. Reinhardt, To Bleach or Not to Bleach – New Oxygen-Based Bleach Technology, in 5th World Conference on Detergents: Reinventing the Industry: Opportunities and Challenges, edit. A. Cahn, AOCS Publishing, 2003, ISBN:978-1-893997-40-0.
- ↑ Clariant Surfactant Division: The Clean and Clever Way of Bleaching, PERACTIVE® (PDF; 885 kB), August 1999.
- ↑ M. Sajitz, J. Grohmann: Hygiene Effects of Bleach Systems in Laundry Detergents[yes|permanent dead link|dead link}}] (PDF; 401 kB), SOFW Journal 10-2012.
- ↑ G. Reinhardt et al., Neue reaktive Bleichaktivatoren – eine Gratwanderung zwischen Bleicheffizienz und Farb-/Faserschädigung, Tenside, Surf. Det., 34 (6), 404-409 (1997)
- ↑ "Ammoniumnitrile und deren Verwendung als Bleichaktivatoren" EP patent 0790244, issued 1997-08-20, assigned to Hoechst AG
- ↑ Lars Cuypers, Martina Hirschen, Gerd Reinhardt (December 2005), "Bleaching Product Development in View of Ecological Aspects" (in German), Tenside Surfactants Detergents 42 (6): 342–346, doi:10.3139/113.100277
- ↑ "Phthalimidoperoxihexansäure, Verfahren zu deren Herstellung und deren Verwendung" EP patent 0349940, issued 1998-05-13, assigned to Clariant GmbH
- ↑ "Color-safe bleach boosters, compositions and laundry methods employing same" EP patent 0923636, issued 20. Januar 1998-01-20, assigned to The Procter & Gamble Co.
- ↑ "Verwendung von cyclischen Zuckerketonen als Katalysator für Persauerstoffverbindungen" EP patent 1209221, issued 2002-05-29, assigned to Clariant GmbH
- ↑ "Verwendung von Übergangsmetallkomplexen mit Oxim-Liganden als Bleichkatalysatoren" EP patent 1225215, issued 2002-07-24, assigned to Clariant GmbH
- ↑ "Bleach activation multinuclear manganese-based coordination complexes" US patent 5244594, issued 1993-09-14, assigned to Lever Brothers Co.
- ↑ M. Verrall, Unilever consigns manganese catalyst to the back-burner, Nature, 373, (1995), 181 und Chemistry in action!, 45, "The soap wars: detergent giants fight dirty". http://www3.ul.ie/~childsp/CinA/Issue45/detergent_giants.htm., letzte Revision am 17. November 1996.
 |