Chemistry:Sodium percarbonate
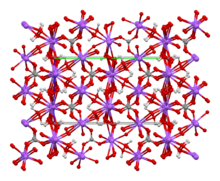 Crystal structure at 100 K [1]
| |
| Names | |
|---|---|
| IUPAC name
sodium carbonate—hydrogen peroxide (2/3)
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3378 |
| |
| |
| Properties | |
| 2 Na 2CO 3 · 3 H 2O 2 | |
| Molar mass | 157.009 g·mol−1 |
| Appearance | White solid |
| Density | 2.01 g/cm3 at 20.4 °C (68.7 °F)[2] |
| 150 g/L at 20 °C (68 °F)[2] | |
| Hazards[2] | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H272, H302, H318, H401 | |
| P210, P220, P264, P280, P301+312+330Script error: No such module "Preview warning".Category:GHS errors, P305+351+338+310, P370+378, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Related compounds | |
Other anions
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium percarbonate or sodium carbonate peroxide is an inorganic compound with the formula 2 Na
2CO
3 · 3 H
2O
2. It is an adduct of sodium carbonate ("soda ash" or "washing soda") and hydrogen peroxide (that is, a perhydrate). It is a colorless, crystalline, hygroscopic, and water-soluble solid.[4] It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.
The product is used in some eco-friendly bleaches and other cleaning products.[4]
History
Sodium percarbonate was first prepared in 1899 by the Russian chemist Sebastian Moiseevich Tanatar (7 October 1849 – 30 November 1917).[5]
Structure
At room temperature, solid sodium percarbonate has the orthorhombic crystal structure, with the Cmca crystallographic space group. The structure changes to Pbca as the crystals are cooled below about −30 °C (−22 °F).[1]
Chemistry
Dissolved in water, sodium percarbonate yields a mixture of hydrogen peroxide, sodium cations (Na+
), and carbonate (CO2−
3).[4][6]
- 2 Na
2CO
3 · 3 H
2O
2 → 3 H
2O
2 + 4 Na+
+ 2 CO2−
3
Production
Sodium percarbonate is produced industrially by crystallization of a solution of sodium carbonate and hydrogen peroxide, with attention to the pH and concentrations.[7][1] This method is also convenient for the laboratory preparation. Alternatively, dry sodium carbonate may be treated directly with concentrated hydrogen peroxide solution.[citation needed]
World production capacity of this compound was estimated at several hundred thousand tons for 2004.[8]
Uses
As an oxidizing agent, sodium percarbonate is an ingredient in a number of home and laundry cleaning products, including non-chlorine bleach products such as Oxyper, OxiClean, Tide laundry detergent,[4] and Vanish.[6]
Sodium percarbonate is also used as a cleaning agent in homebrewing.[9]
Sodium percarbonate can be used in organic synthesis as a convenient source of anhydrous H
2O
2, in particular in solvents that cannot dissolve the carbonate but can leach the H
2O
2 out of it.[10] A method for generating trifluoroperacetic acid in situ for use in Baeyer–Villiger oxidations from sodium percarbonate and trifluoroacetic anhydride has been reported; it provides a convenient and cheap approach to this reagent without the need to obtain highly concentrated hydrogen peroxide.[11][12]
References
- ↑ 1.0 1.1 1.2 Pritchard, R.G.; Islam, E. (2003). "Sodium percarbonate between 293 and 100 K". Acta Crystallographica Section B B59 (5): 596–605. doi:10.1107/S0108768103012291. PMID 14586079.
- ↑ 2.0 2.1 2.2 Sigma-Aldrich Co., Sodium percarbonate.
- ↑ "SDS - Sodium percarbonate". Thermo-Fisher Scientific. 26 March 2024. p. 3. https://www.fishersci.com/store/msds?partNumber=AC370735000&countryCode=US&language=en.
- ↑ 4.0 4.1 4.2 4.3 Craig W. Jones (1999). Applications of Hydrogen Peroxide and Derivatives. Royal Society of Chemistry. ISBN 0-85404-536-8.
- ↑ Tanatar, S. (1899). "Percarbonate" (in German). Berichte der Deutschen Chemischen Gesellschaft zu Berlin 32 (2): 1544–1546. doi:10.1002/cber.18990320233. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1i1w&view=1up&seq=198&skin=2021.
- ↑ 6.0 6.1 "Oxygen-based bleaches[Usurped!]", The Royal Society of Chemistry, and Reckitt Benckiser (the manufacturers of Vanish).
- ↑ Adams, J. M.; Pritchard, R. G. (1 December 1977). "The crystal structure of sodium percarbonate: an unusual layered solid". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 33 (12): 3650–3653. doi:10.1107/S0567740877011790.
- ↑ Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2.
- ↑ "Sodium Percarbonate". https://www.morebeer.com/products/sodium-percarbonate.html. Retrieved 26 June 2020.
- ↑ McKillop, A (1995). "Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis". Tetrahedron 51 (22): 6145–6166. doi:10.1016/0040-4020(95)00304-Q.
- ↑ Gang, Ho Jeong; Jeong, Hui Seon (January 1996). "New Method of Generating Trifluoroperoxy-acetic acid for the Baeyer-Villiger Reaction". Bulletin of the Korean Chemical Society 17 (1): 5–6. doi:10.5012/bkcs.1996.17.1.5. ISSN 1229-5949. https://koreascience.kr/article/JAKO199613464464680.pdf.
- ↑ Caster, Kenneth C.; Rao, A. Somasekar; Mohan, H. Rama; McGrath, Nicholas A.; Brichacek, Matthew (2012). "Encyclopedia of Reagents for Organic Synthesis". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt254.pub2. ISBN 978-0471936237.
External links
- Organic Chemistry Portal: Sodium percarbonate
- Consumer Product Information Database: Sodium percarbonate
 |

