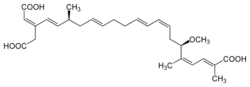
Chemistry:Bongkrek acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,4Z,6R,8Z,10E,14E,17S,18E,20Z)-20-(Carboxymethyl)-6-methoxy-2,5,17-trimethyldocosa-2,4,8,10,14,18,20-heptaenedioic acid | |
| Other names
Bongkrekic acid
Bongkrekik acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Bongkrekic+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H38O7 | |
| Molar mass | 486.605 g·mol−1 |
| Appearance | Odorless and colorless |
| Melting point | 50 to 60 °C (122 to 140 °F; 323 to 333 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bongkrek acid (also known as bongkrekic acid[1]) is a respiratory toxin produced in fermented coconut or corn contaminated by the bacterium Burkholderia gladioli pathovar cocovenenans.[2][3][4] It is a highly toxic, heat-stable, colorless, odorless, and highly unsaturated tricarboxylic acid that inhibits the ADP/ATP translocase, also called the mitochondrial ADP/ATP carrier, preventing ATP from leaving the mitochondria to provide metabolic energy to the rest of the cell.[4][5] Bongkrek acid, when consumed through contaminated foods, mainly targets the liver, brain, and kidneys along with symptoms that include vomiting, diarrhea, urinary retention, abdominal pain, and excessive sweating.[4] Most of the outbreaks are found in Indonesia and China where fermented coconut and corn-based foods are consumed.
Discovery and history
In 1895, there was a food-poisoning outbreak in Java, Indonesia. The outbreak was caused by the consumption of Indonesian traditional food called tempe Bongkrek. During this time, tempe Bongkrek served as a main source of protein in Java due to its inexpensiveness. Tempe Bongkrek is made by extracting the coconut meat by-product from coconut milk into a form of cake, which is then fermented with R. oligosporus mold.[4][1] The first outbreak of the Bongkrek poisoning by tempe Bongkrek was recorded by Dutch researchers; however no further research to find the cause of the poisoning was conducted in 1895.[6] During 1930s, Indonesian government went through an economic depression, and this condition caused some of the people to make tempe Bongkrek by themselves, instead of buying it directly from well-trained producers. As a result, the poisonings occurred frequently, reaching 10 to 12 a year. Dutch scientists, named W.K Mertens and A.G. van Veen from the Eijkman Institute of Jakarta, started to find the cause of the poisoning in the early 1930s. They successfully identified the source of poisoning, which was a bacterium called Pseudomonas cocovenenans .[6][7] This bacterium, which is also named Burkholderia cocovenenans , caused the synthesis of a poisonous substance called Bongkrek acid. B. cocovenenans is commonly found in plants and soil, which can be taken up by coconuts and corn, leading to the synthesis of Bongkrek acid during the fermentation of such foods.[6] Since 1975, consumption of contaminated tempe Bongkrek has caused more than 3000 cases of Bongkrek acid poisoning.[4] In Indonesia, the overall reported mortality rate has turned out to be 60%. Due to the severity of the situation, the production of tempe Bongkrek has been banned since 1988.[4][6]
Synthesis
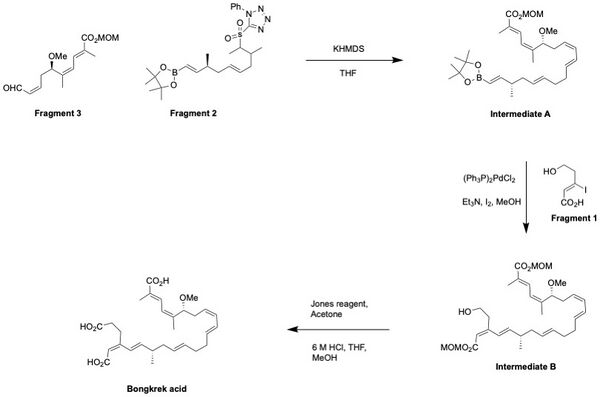
There were multiple attempts to synthesize Bongkrek acid using different numbers of fragments since the first total synthesis of the acid by E.J. Corey in 1984.[8] One of the unique attempts to synthesize Bongkrek acid was done by Shindo's group from Kyushu University in 2009. Unlike other attempts such as the one from Lev's group,[9] Shindo's group used three fragments to synthesize Bongkrek acid.[10]
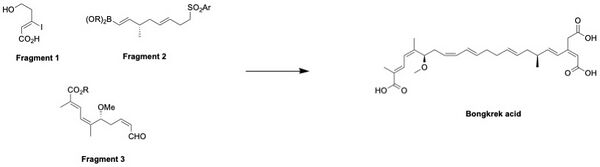
The Fragments 1, 2, and 3 were individually synthesized in the lab.[10] After the synthesis of each fragment required for Bongkrek acid synthesis, the fragments 2 and 3 were first coupled together through Julia olefination in the presence of KHMDS. The resulting intermediate, abbreviated as A in the scheme below, was then coupled with the fragment 1 through Suzuki coupling. After forming intermediate B, Bongkrek acid was finally synthesized by treating it with methanol (primary alcohol) through Jones reagent and acid deprotection of the methoxymethyl ester. The first total synthesis of Bongkrek acid by E.J, Corey required 32 steps;[8] however Shindo successfully reduced the steps into a total of 18 steps by efficiently utilizing Julia olefination and Suzuki coupling along with a higher yield by 6.4%.[10]
Mechanism of action
Adenine nucleotide translocator, abbreviated as ANT, provides ATP from mitochondria to the cytosol in exchanging of cytosolic ADP. The way Bongkrek acid works is that it interrupts the transport process of the cytosolic ADP in the inner membrane of mitochondria by inhibiting the mitochondrial ANT. The interesting part of this inner membrane of mitochondria is that the ANT forms the internal membrane channel of the mitochondrial permeability transition pore, known as MPTP. Bongkrek acid is permeable through this membrane and binds to the surface of ANT, inhibiting ANT’s translocation.[11] Once Bongkrek acid binds to the surface of ANT, the acid forms hydrogen bonding interactions with ANT protein residues. The hydrogen bonding interactions are mainly formed with the oxygens from the carboxylic acid fragments of Bongkrek acid. The most prominent contribution to the hydrogen bonding interaction comes from the interaction with the side chain amino group, Arg-197. Another prominent contribution of binding Bongkrek acid with ANT is the electrostatic interaction between the acid and the ANT’s amino acid, Lys-30. As a result, the hydrogen bonding interactions and the salt bridge (protein and supramolecular) put Bongkrek acid in the center of the ANT active site, inhibiting the action of the translocase.[11]
Mitochondrial synthesis of ATP requires ADP transport from the cytosol into the mitochondrial matrix through the ANT, meaning it plays a critical role in providing energy for the cells in the first place. ADP/ATP exchange heavily depends on the transition between two distinct conformation states of ANT: cytosolic state (c-state) and matrix state (m-state). In the c-state, the active site of ANT faces toward the cytosol, where it attracts the cytosol ADP, and in the m-state, the active site of ANT faces toward the mitochondrial matrix, where it can release the cytosol ADP and attracts the synthesized ATP. The interaction between the acid and the ANT causes the conformational change of the ANT. Bongkrek acid locks ANT in the m-state. The structure of Bongkrek acid-ANT shows that there are six transmembrane alpha helices covering up the active site of the ANT, preventing the binding of adenosine nucleotides. This means ANT can’t receive ADP from the cytosol, ultimately preventing the synthesis of ATP.[11][12]
Symptoms of poisoning and treatments
After consumption of Bongkrek acid-contaminated corn-based or coconut-based foods, the latency period is expected to be between 1 and 10 hours. The symptoms of Bongkrek acid poisoning are like other mitochondrial toxins. The common symptoms of Bongkrek acid poisoning are dizziness, somnolence, excessive sweating, palpitations, abdominal pain, vomiting, diarrhea, hematochezia, hematuria, and urinary retention. The death usually occurs after 1 to 20 hours after the onset of the symptoms of Bongkrek acid poisoning.[4] Another common symptom of Bongkrek acid poisoning is limb soreness. In the first reported BA poisoning case in Africa, 12/17 people were reported to have limb soreness as one of their main symptoms.[13] A fatal dose for humans can be as low as 1 to 1.5 mg, and other source also states that oral LD50 is 3.16 mg per kg body weight.[4]
Due to lack of studies on the toxicokinetics of Bongkrek acid, there are no specific treatments or antidotes for Bongkrek acid. The commonly used protocol to treat Bongkrek acid poisoning is to remove the toxins that aren't absorbed by the ANT and to provide treatments that are specific to the symptoms that patients are having. Due to the lack of specific treatments and antidotes for the toxins, the timing is critical to reverse the severe physiological effects.[14]
References
- ↑ 1.0 1.1 Garcia, R. A.; Hotchkiss, J. H.; Steinkraus, K. H. (1999). "The Effect of Lipids on Bongkrekic (Bongkrek) Acid Toxin Production by Burkholderia cocovenenans in Coconut Media". Food Additives and Contaminants 16 (2): 63–69. doi:10.1080/026520399284217. PMID 10435074.
- ↑ Henderson, P. J. F.; Lardy, H. A. (1970). "Bongkrekic Acid: An Inhibitor of Adenine Nucleotide Translocase of Mitochondria". Journal of Biological Chemistry 245 (6): 1319–1326. doi:10.1016/S0021-9258(18)63238-7. PMID 4245638. http://www.jbc.org/content/245/6/1319.full.pdf. Retrieved 2013-01-20.
- ↑ De Bruijn, J.; Frost, D. J.; Nugteren, D. H.; Gaudemer, A.; Lijmbach, G. W. M.; Cox, H. C.; Berends, W. (1973). "Structure of Bongkrekic Acid". Tetrahedron 29 (11): 1541–1547. doi:10.1016/S0040-4020(01)83395-0.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Anwar, Mehruba; Kasper, Amelia; Steck, Alaina R.; Schier, Joshua G. (June 2017). "Bongkrekic Acid—a Review of a Lesser-Known Mitochondrial Toxin". Journal of Medical Toxicology 13 (2): 173–179. doi:10.1007/s13181-016-0577-1. ISSN 1556-9039. PMID 28105575.
- ↑ Toxicants Occurring Naturally in Foods. National Academy of Sciences. 1973. p. 472. ISBN 9780309021173. https://archive.org/details/toxicantsoccurri0000unse.
- ↑ 6.0 6.1 6.2 6.3 Riyanto, Rifqi Ahmad (2019). "A Short Review of Bongkrek Acid In Food Safety Perspective". Food ScienTech Journal 1: 65–68. doi:10.33512/fsj.vli2.6427. https://www.researchgate.net/profile/Rifqi-Riyanto/publication/339683533_A_SHORT_REVIEW_OF_BONGKREKIC_ACID_IN_FOOD_SAFETY_PERSPECTIVE/links/5e7033d492851c1a689a6dc8/A-SHORT-REVIEW-OF-BONGKREKIC-ACID-IN-FOOD-SAFETY-PERSPECTIVE.pdf?_sg%5B0%5D=started_experiment_milestone&origin=journalDetail. Retrieved 29 April 2023.
- ↑ "A Short Review of Bongkrekic Acid In Food Safety Perspective. [Abstract"]. https://www.researchgate.net/publication/339683533_A_SHORT_REVIEW_OF_BONGKREKIC_ACID_IN_FOOD_SAFETY_PERSPECTIVE/fulltext/5e5fa6c34585152ce806be7b/A-SHORT-REVIEW-OF-BONGKREKIC-ACID-IN-FOOD-SAFETY-PERSPECTIVE.pdf.
- ↑ 8.0 8.1 Corey, E. J.; Tramontano, Alfonso (January 1984). "Total synthesis of bongkrekic acid". Journal of the American Chemical Society 106 (2): 462–463. doi:10.1021/ja00314a056.
- ↑ Francais, Antoine; Leyva, Antonio; Etxebarria-Jardi, Gorka; Ley, Steven V. (15 January 2010). "Total Synthesis of the Anti-Apoptotic Agents Iso- and Bongkrekic Acids". Organic Letters 12 (2): 340–343. doi:10.1021/ol902676t.
- ↑ 10.0 10.1 10.2 Sato, Yukiko; Aso, Yoshifumi; Shindo, Mitsuru (July 2009). "Efficient synthesis of bongkrekic acid. Three-component convergent strategy". Tetrahedron Letters 50 (28): 4164–4166. doi:10.1016/j.tetlet.2009.04.129.
- ↑ 11.0 11.1 11.2 Li, Hongmei; Liang, Zhen; Li, Ying; Wen, Jiazhen; Zhang, Rong (February 2023). "Molecular docking and molecular dynamics simulation study on the toxicity mechanism of bongkrekic acid". Toxicon 223: 107021. doi:10.1016/j.toxicon.2023.107021.
- ↑ Temkin, Vladislav; Huang, Qiquan; Liu, Hongtao; Osada, Hiroyuki; Pope, Richard M. (1 March 2006). "Inhibition of ADP/ATP Exchange in Receptor-Interacting Protein-Mediated Necrosis". Molecular and Cellular Biology 26 (6): 2215–2225. doi:10.1128/MCB.26.6.2215-2225.2006.
- ↑ Huang, Qingyu; Wu, Zhentian (2021). "Safe Eating of Fermented Corn and Coconut Food: Mechanism, Clinical Manifestations and Inhibition of Food Poisoning Involved in Bongkrekic Acid". E3S Web of Conferences 267: 02075. doi:10.1051/e3sconf/202126702075.
- ↑ Yuan, Yuan; Gao, Rui; Liang, Qiang; Song, Li; Huang, Jun; Lang, Nan; Zhou, Jing (18 December 2020). "A Foodborne Bongkrekic Acid Poisoning Incident — Heilongjiang Province, 2020". China CDC Weekly 2 (51): 975–978. doi:10.46234/ccdcw2020.264.
 |

