Chemistry:Boscalid
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| Preferred IUPAC name
2-chloro-N-(4'-chloro[1,1'-biphenyl]-2-yl)pyridine-3-carboxamide | |
| Other names
BAS 510F, Nicobifen
| |
| Identifiers | |
3D model (JSmol)
|
|
| 10092464 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1] | |
| C18H12Cl2N2O | |
| Molar mass | 343.21 |
| Appearance | White to beige coloured crystalline powder |
| Density | 1.381 g/cm3 |
| Melting point | 143°C |
| 4.6 mg/L (20 °C) | |
| log P | 2.96 |
| Hazards | |
| GHS pictograms | 
|
| HH411Script error: No such module "Preview warning".Category:GHS errors | |
| P273, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Boscalid is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by BASF in 2002 using their brand name Endura.[3] The compound is an biphenyl amide derived inhibitor of succinate dehydrogenase.[4]
History
Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. The first compound in this class was carboxin, which had a narrow spectrum of useful biological activity, mainly on basidiomycetes and was used as a seed treatment. Many companies made analogues with the aim of expanding the range of species controlled and boscalid was successful in doing so.[5][6]
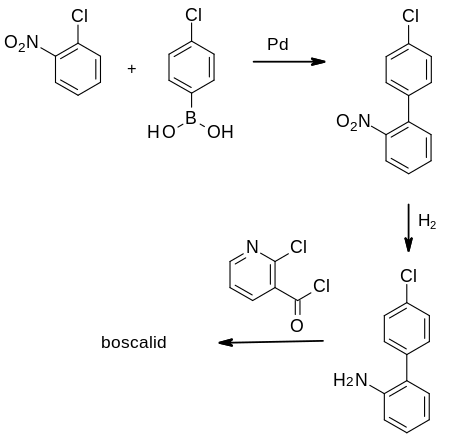
Synthesis
The first synthesis of boscalid was disclosed in patents filed by BASF in 1995.[7][8][9]
The aminobiphenyl required for reaction with the acid chloride of 2-chloronicotinic acid is prepared in two steps. The first is a palladium-catalysed Suzuki reaction, followed by reduction. As the final product has been manufactured at the multi-tonne scale, considerable efforts have been made to optimise these reactions.[8][9]
Mechanism of action
Succinate dehydrogenase inhibitors (SDHI) of this type act by binding at the quinone reduction site of the enzyme complex, preventing ubiquinone from doing so. As a consequence, the tricarboxylic acid cycle and electron transport chain cannot function.[10][11]
Usage
Boscalid has fungicidal effects against a wide range of crop pests. These include Alternaria, grey mold (Botrytis cinerea), white mold (Sclerotinia sclerotiorum), and powdery mildew (Uncinula necator). As a result, it has use in crops including fruits, soybeans and vegetables.[3][5]
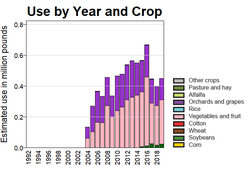
The estimated annual use of boscalid in US agriculture is mapped by the US Geological Survey and shows that it is mainly applied to fruit crops, with a maximum use of over 600,000 pounds (270,000 kg) in 2016.[12] Global sales in 2014 were estimated at $390 million. The compound lacks full control of important cereal diseases, especially septoria leaf blotch Zymoseptoria tritici, which has limited its potential.[5]
Human safety
Boscalid has low acute toxicity:[1] the Codex Alimentarius database maintained by the FAO lists the maximum residue limits for it in various food products.[13]
Environmental effects
The compound is very persistent in field conditions[1] and its environmental fate and consequent ecotoxicology have been reviewed.[4]
Resistance management
Fungal populations have the ability to develop resistance to SDHI inhibitors.[14] This potential can be mitigated by careful management. Reports of individual pest species becoming resistant[1] are monitored by manufacturers, regulatory bodies such as the EPA and the Fungicides Resistance Action Committee (FRAC).[15] The risks of resistance developing can be reduced by using a mixture of two or more fungicides which each have activity on relevant pests but with unrelated mechanisms of action. FRAC assigns fungicides into classes so as to facilitate this.[16]
Brands
Boscalid is the ISO common name for the active ingredient which is formulated into the branded product sold to end-users. It was also known as nicobifen.[17] Endura and Emerald are the brand names first used by BASF's formulations[3] but the compound has subsequently been sold under a range of product names.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Pesticide Properties Database (2023-07-25). "Boscalid". University of Hertfordshire. http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/86.htm.
- ↑ "GHS Classification". 2023-07-22. https://pubchem.ncbi.nlm.nih.gov/compound/56933411#section=Safety-and-Hazards.
- ↑ 3.0 3.1 3.2 "Endura fungicide". EPA. 2002-11-08. https://www3.epa.gov/pesticides/chem_search/ppls/007969-00197-20221108.pdf.
- ↑ 4.0 4.1 Yibing, He (2006). "Boscalid". FAO. https://www.fao.org/fileadmin/user_upload/IPM_Pesticide/JMPR/Evaluations/2006/Boscalid.pdf.
- ↑ 5.0 5.1 5.2 Walter, Harald (2016). "Fungicidal Succinate-Dehydrogenase-Inhibiting Carboxamides". Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 405–425. doi:10.1002/9783527693931.ch31. ISBN 9783527339471.
- ↑ "History of SDHI-fungicides". https://www.frac.info/frac-teams/working-groups/sdhi-fungicides/information.
- ↑ ; Goetz, N & Harreus, A et al."Anilide derivatives and their use for combating botrytis" US patent 5589493, published 1996-12-31, assigned to BASF SE
- ↑ 8.0 8.1 Glasnov, Toma N.; Kappe, C. Oliver (2010). "Toward a Continuous-Flow Synthesis of Boscalid". Advanced Synthesis & Catalysis 352 (17): 3089–3097. doi:10.1002/adsc.201000646. https://www.academia.edu/download/54413923/adsc.20100064620170912-2977-1mb75lu.pdf.
- ↑ 9.0 9.1 Takale, Balaram S.; Thakore, Ruchita R.; Mallarapu, Rushil; Gallou, Fabrice; Lipshutz, Bruce H. (2020). "A Sustainable 1-Pot, 3-Step Synthesis of Boscalid Using Part per Million Level Pd Catalysis in Water". Organic Process Research & Development 24: 101–105. doi:10.1021/acs.oprd.9b00455. https://par.nsf.gov/servlets/purl/10167085.
- ↑ Oyedotun, Kayode S.; Lemire, Bernard D. (2004). "The Quaternary Structure of the Saccharomyces cerevisiae Succinate Dehydrogenase". Journal of Biological Chemistry 279 (10): 9424–9431. doi:10.1074/jbc.M311876200. PMID 14672929.
- ↑ Avenot, Hervé F.; Michailides, Themis J. (2010). "Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi". Crop Protection 29 (7): 643–651. doi:10.1016/j.cropro.2010.02.019.
- ↑ US Geological Survey (2021-10-12). "Estimated Agricultural Use for boscalid, 2019". https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2019&map=BOSCALID&hilo=L&disp=Boscalid.
- ↑ FAO / WHO. "Boscalid". https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=221.
- ↑ Cosseboom, Scott; Hu, Mengjun (2021). "Identification and Characterization of Fungicide Resistance in Botrytis Populations from Small Fruit Fields in the Mid-Atlantic United States". Plant Disease 105 (9): 2366–2373. doi:10.1094/pdis-03-20-0487-re. PMID 33719541.
- ↑ "Fungicides Resistance Action Committee website". https://www.frac.info/.
- ↑ "Search Fungicides to find FRAC Recommendations". https://www.frac.info/fungicide-resistance-management/by-fungicide-common-name.
- ↑ "Compendium of Pesticide Common Names: Boscalid". BCPC. http://www.bcpcpesticidecompendium.org/boscalid.html.
External links
- PPDB pesticides properties database entry for boscalid
 |