Chemistry:BrMT
| |
| Names | |
|---|---|
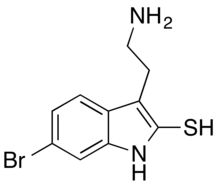
| IUPAC name
3-(2-Aminoethyl)-6-bromo-1H-indole-2-thiol
| |
| Other names
6-Bromo-2-mercaptotryptamine
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H11BrN2S | |
| Molar mass | 271.18 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
BrMT (6-bromo-2-mercaptotryptamine) is a neurotoxin found in the hypobranchial gland of the marine snail species Calliostoma canaliculatum. The disulfide-linked dimer of BrMT possesses inhibitory effects on the Kv1 and Kv4 families of voltage-gated potassium channels.
Source
BrMT was first isolated from the mucus of Calliostoma canaliculatum, a cone snail found in the temperate coastal waters of the western Pacific. BrMT is the first compound found in the hypobranchial gland mucus to produce a biological response.[1]
Chemistry
BrMT is a brominated tryptamine. It has a thiol group, allowing dimerization via a disulfide linkage.[1] BrMT is found to be light-sensitive and unstable in a reducing environment. Its first total synthesis was reported in 2013.[2]
Target
Calliostoma canaliculatum deters predators by covering its shells with BrMT-containing mucus, in particular when exposed to a predator, such as Pycnopodia helianthoides or Pisaster giganteus.[3]
The BrMT dimer is known to affect voltage-gated potassium channels in the central nervous system. It strongly inhibits ShBΔ potassium channels, and to a lesser degree also isoforms found in humans (hKv1.1) and squid (sqKv1.A). It also affects members of Kv4 family (Kv4.1 and Kv4.2) and Drosophila ether-à-go-go channels.[1]
Mode of action
The mode of action of BrMT involves inhibition of specific voltage gated potassium channels present in the nervous system. By stabilizing the voltage sensor of the ion channel, the opening of ShBΔ ion channels and other Kv1 members is inhibited.[2] BrMT has an Template:IC50 of 1.1 ± 0.1 μM on ShBΔ channels, a member of the Kv1 family.[4] The BrMT binding to ShBΔ has been found to be allosteric in nature, due to a change of conformation in the K+ channel subunits and not by blocking the entrance of the channel.[4][5]
References
- ↑ 1.0 1.1 1.2 Kelley, Wayne P.; Wolters, Andrew M.; Sack, Jon T.; Jockusch, Rebecca A.; Jurchen, John C.; Williams, Evan R.; Sweedler, Jonathan V.; Gilly, William F. (September 2003). "Characterization of a Novel Gastropod Toxin (6-Bromo-2-mercaptotryptamine) That Inhibits Shaker K Channel Activity". Journal of Biological Chemistry 278 (37): 34934–34942. doi:10.1074/jbc.m301271200. ISSN 0021-9258. PMID 12815055.
- ↑ 2.0 2.1 Gao, Detian; Sand, Rheanna; Fu, Hao; Sharmin, Nazlee; Gallin, Warren J.; Hall, Dennis G. (2013-10-15). "Synthesis of the non-peptidic snail toxin 6-bromo-2-mercaptotryptamine dimer (BrMT)2, its lower and higher thio homologs and their ability to modulate potassium ion channels". Bioorganic & Medicinal Chemistry Letters 23 (20): 5503–5506. doi:10.1016/j.bmcl.2013.08.070. ISSN 0960-894X. PMID 24021461.
- ↑ Bryan, Patrick J.; Mcclintock, James B.; Hamann, Mark (1997-03-01). "Behavioral and Chemical Defenses of Marine Prosobranch Gastropod Calliostoma canaliculatum in Response to Sympatric Seastars" (in en). Journal of Chemical Ecology 23 (3): 645–658. doi:10.1023/B:JOEC.0000006401.97339.b9. ISSN 1573-1561.
- ↑ 4.0 4.1 Dockendorff, Chris; Gandhi, Disha M.; Kimball, Ian H.; Eum, Kenneth S.; Rusinova, Radda; Ingólfsson, Helgi I.; Kapoor, Ruchi; Peyear, Thasin et al. (2018-05-08). "Synthetic Analogues of the Snail Toxin 6-Bromo-2-mercaptotryptamine Dimer (BrMT) Reveal That Lipid Bilayer Perturbation Does Not Underlie Its Modulation of Voltage-Gated Potassium Channels" (in en). Biochemistry 57 (18): 2733–2743. doi:10.1021/acs.biochem.8b00292. ISSN 0006-2960. PMID 29616558.
- ↑ Sack, Jon T.; Aldrich, Richard W. (2006-06-26). "Binding of a Gating Modifier Toxin Induces Intersubunit Cooperativity Early in the Shaker K Channel's Activation Pathway". The Journal of General Physiology 128 (1): 119–132. doi:10.1085/jgp.200609492. ISSN 1540-7748. PMID 16801385.
 |


