Chemistry:Bromocresol purple
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
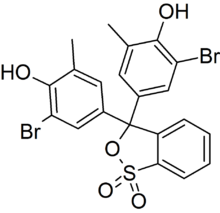
3,3-Bis(3-bromo-4-hydroxy-5-methylphenyl)-2,1λ6-benzoxathiole-1,1(3H)-dione | |
| Other names
5′,5′′-Dibromo-o-cresolsulfonephthalein
Bromcresol purple | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H16Br2O5S | |
| Molar mass | 540.22 g·mol−1 |
| Appearance | Purple powder |
| Melting point | 241 to 242 °C (466 to 468 °F; 514 to 515 K) (decomposition) |
| < 0.1 % | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromocresol purple (BCP) or 5′,5″-dibromo-o-cresolsulfophthalein, is a dye of the triphenylmethane family (triarylmethane dyes) and a pH indicator. It is colored yellow below pH 5.2, and violet above pH 6.8. In its cyclic sulfonate ester form, it has a pKa value of 6.3, and is usually prepared as a 0.04% aqueous solution.[1]
Uses
| Bromocresol purple (pH indicator) | ||
| below pH 5.2 | above pH 6.8 | |
| 5.2 | ⇌ | 6.8 |
Bromocresol purple is used in medical laboratories to measure albumin. Use of BCP in this application may provide some advantage over older methods using bromocresol green.[2][3] In microbiology, it is used for staining dead cells based on their acidity, and for the isolation and assaying of lactic acid bacteria.[4][5]
In photographic processing, it can be used as an additive to acid stop baths to indicate that the bath has reached neutral pH and needs to be replaced.[6]
Bromocresol purple milk solids glucose agar is used as a medium used to distinguish dermatophytes from bacteria and other organisms in cases of ringworm fungus (T. verrucosum) infestation in cattle and other animals.[7][8]
pH Indicator
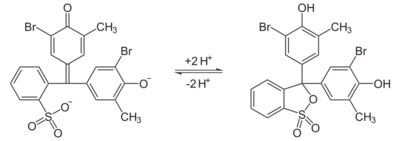
Similar to bromocresol green, the structure of bromocresol purple changes with pH. Changing the level of acidity causes a shift in the equilibrium between two different structures that have different colors. In near-neutral or alkaline solution, the chemical has a sulfonate structure that gives the solution a purple color. As the pH decreases, it converts to a sultone (cyclic sulfonic ester) that colors the solution yellow. In some microbiology tests, this change is used as an indicator of bacterial growth.[9][10]
See also
References
- ↑ "Bromocresol Purple". NCBI PubChem. National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8273.
- ↑ Bachmann, Lorin M.; Yu, Min; Boyd, James C.; Bruns, David E.; Miller, W. Greg (2017-03-01). "State of Harmonization of 24 Serum Albumin Measurement Procedures and Implications for Medical Decisions" (in en). Clinical Chemistry 63 (3): 770–779. doi:10.1373/clinchem.2016.262899. ISSN 0009-9147. PMID 28073902.
- ↑ Ito, Shigenori; Yamamoto, Daisuke (2010-02-02). "Mechanism for the color change in bromocresol purple bound to human serum albumin". Clinica Chimica Acta 411 (3): 294–295. doi:10.1016/j.cca.2009.11.019. PMID 19932090.
- ↑ Kurzweilová, H.; Sigler, K. (November 1993). "Fluorescent staining with bromocresol purple: a rapid method for determining yeast cell dead count developed as an assay of killer toxin activity.". Yeast 9 (11): 1207–1211. doi:10.1002/yea.320091107. PMID 7509098.
- ↑ Lee, H.M.; Lee, Y. (June 2008). "A differential medium for lactic acid-producing bacteria in a mixed culture". Letters in Applied Microbiology 46 (6): 676–681. doi:10.1111/j.1472-765X.2008.02371.x. PMID 18444977.

- ↑ Anchell, Steve (2016). The Darkroom Cookbook (4 ed.). Routledge. ISBN 9781317337607. https://books.google.com/books?id=u67OCwAAQBAJ&pg=PT622.
- ↑ Kane, J.; Summerbell, R.; Sigler, L.; Krajden, S.; Land, G. (1997). Laboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails. Belmont, CA: Star Publishing Company. ISBN 9780898631579.
- ↑ Beneke, E. S.; Rogers, A. L. (1996). Medical Mycology and Human Mycoses (illustrated ed.). Belmont, CA: Star Publishing Company. pp. 85–90. ISBN 9780898631753.
- ↑ "Bromocresol Purple - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/bromocresol-purple.
- ↑ "Archived copy". https://www.thomassci.com/FetchFile.ashx?id=2807195c-9bfc-46a6-b088-61dd6b1f3e42.
External links
 |