Chemistry:Buparlisib
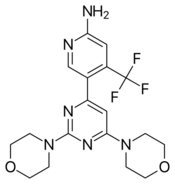 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H21F3N6O2 |
| Molar mass | 410.39 g/mol |
| 3D model (JSmol) | |
| |
Buparlisib (INN,[1] codenamed BKM120) is an investigational small molecule orally-available pan-class I phosphoinositide 3-kinase inhibitor.[2]
Clinical trials
In December 2015 it is reporting results for the phase III BELLE-2 clinical trial for advanced HR+/HER2 endocrine-resistant breast cancer.[3] Encouraging results are reported in some sub-populations — e.g., some PI3K mutations.[3]Lawrence, Leah (11 December 2015). "Buparlisib Benefits Women With PIK3CA Mutations in Circulating Tumor DNA". Cancer Network. http://www.cancernetwork.com/sabcs-2015/buparlisib-benefits-women-pik3ca-mutations-circulating-tumor-dna.</ref>
A Phase Ib clinical trial combined buparlisib and letrozole in the treatment of estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic cancer. Results indicated that the drug combination was safe on two different treatment schedules and the clinical benefit rate was 31%. Common toxicities included hyperglycemia, nausea, fatigue, transaminitis, and mood disorders, though the toxicities are reversible and well tolerated.[4]
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 68". World Health Organization. p. 304. https://www.who.int/medicines/publications/druginformation/issues/RL_68.pdf.
- ↑ "Buparlisib , an oral pan-PI3K inhibitor for the treatment of breast cancer". Expert Opinion on Investigational Drugs 24 (3): 421–31. March 2015. doi:10.1517/13543784.2015.1008132. PMID 25645727.
- ↑ 3.0 3.1 Johnson, Kate (14 December 2015). "PI3K Inhibitor Penetrates Endocrine-Resistant Breast Cancer". MedScape. http://www.medscape.com/viewarticle/855908.
- ↑ "Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer". Journal of Clinical Oncology 32 (12): 1202–9. April 2014. doi:10.1200/JCO.2013.54.0518. PMID 24663045.
External links
- Buparlisib Shows Promise in Metastatic Breast Cancer. Aug 2014 Buparlisib combined with letrozole
- Phase II Study of Buparlisib + Docetaxel in Advanced or Metastatic Squamous Non-small Cell Lung Cancer (NSCLC) Patients completed

