Chemistry:Pentafluorophenol
From HandWiki
(Redirected from Chemistry:C6F5)
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentafluorophenol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 6F 5OH | |
| Molar mass | 184.065 g·mol−1 |
| Appearance | white solid or colorless liquid |
| Melting point | 32.8 °C (91.0 °F; 305.9 K) |
| Boiling point | 145.6 °C (294.1 °F; 418.8 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H312, H314, H315, H319, H335 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
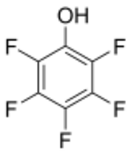
Pentafluorophenol is the organofluorine compound (specifically a fluoroalcohol) with the formula C
6F
5OH. This is the perfluorinated analogue of phenol. It is a white odorless solid that melts just above room temperature. With a pKa of 5.5, it is one of the most acidic phenols.
Uses
Pentafluorophenol is used to prepare pentafluorophenyl esters, which are active esters useful in peptide synthesis.[1]
Environmental hazards
Pentafluorophenol is considered hazardous because of oral, dermal and inhalation toxicity and because it causes severe skin burns and eye damage.[2][3]
References
- ↑ "Pentafluorophenol". Encyclopedia of Reagents for Organic Synthesis. 2009. pp. 1-9. doi:10.1002/047084289X.
- ↑ "Pentafluorophenol SAFETY DATA SHEET". Thermo Fisher Scientific. January 18, 2018. https://www.fishersci.com/store/msds?partNumber=AC147130050&productDescription=PENTAFLUOROPHENOL+99%2B%25+5GR&vendorId=VN00032119&countryCode=US&language=en.
- ↑ "Pentafluorophenol". National Center for Biotechnology Information. February 20, 2021. https://pubchem.ncbi.nlm.nih.gov/compound/13041#section=Hazards-Identification.
 |
