Chemistry:CCK-4
 | |
| Clinical data | |
|---|---|
| Other names | Tetragastrin; Cholecystokinin tetrapeptide |
| Routes of administration | IV |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Metabolism | plasma protease enzymes |
| Elimination half-life | 13 minutes |
| Excretion | N/A |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C29H35N5O7S |
| Molar mass | 597.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
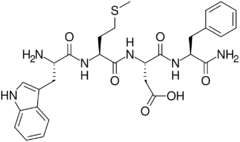
Cholecystokinin tetrapeptide (CCK-4, tetragastrin, Trp-Met-Asp-Phe-NH2) is a peptide fragment derived from the larger peptide hormone cholecystokinin. Unlike cholecystokin which has a variety of roles in the gastrointestinal system as well as central nervous system effects, CCK-4 acts primarily in the brain as an anxiogenic, although it does retain some GI effects, but not as much as CCK-8 or the full length polypeptide CCK-58.
CCK-4 reliably causes severe anxiety symptoms when administered to humans in a dose of as little as 50 μg,[1] and is commonly used in scientific research to induce panic attacks for the purpose of testing new anxiolytic drugs.[2][3][4][5] Since it is a peptide, CCK-4 must be administered by injection, and is rapidly broken down once inside the body so has only a short duration of action,[6] although numerous synthetic analogues with modified properties are known.[7][8][9][10][11][12][13][14][15][16][17]
See also
References
- ↑ "Panic induction with cholecystokinin-tetrapeptide (CCK-4) Increases plasma concentrations of the neuroactive steroid 3alpha, 5alpha tetrahydrodeoxycorticosterone (3alpha, 5alpha-THDOC) in healthy volunteers". Neuropsychopharmacology 30 (1): 192–5. January 2005. doi:10.1038/sj.npp.1300572. PMID 15467707.
- ↑ "Neurobiological investigations into the role of cholecystokinin in panic disorder". Journal of Psychiatry & Neuroscience 18 (4): 178–88. July 1993. PMID 8104032.
- ↑ "Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety". NeuroImage 31 (3): 1197–208. July 2006. doi:10.1016/j.neuroimage.2006.01.035. PMID 16600640.
- ↑ "Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study". Psychopharmacology 192 (4): 479–87. July 2007. doi:10.1007/s00213-007-0738-7. PMID 17318504.
- ↑ "Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers". Human Brain Mapping 30 (2): 511–22. February 2009. doi:10.1002/hbm.20522. PMID 18095276.
- ↑ "Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro". Regulatory Peptides 4 (3): 127–39. August 1982. doi:10.1016/0167-0115(82)90080-5. PMID 6291099.
- ↑ "Structure-based design of new constrained cyclic agonists of the cholecystokinin CCK-B receptor". Journal of Medicinal Chemistry 40 (5): 647–58. February 1997. doi:10.1021/jm9603072. PMID 9057851.
- ↑ "Replacement of glycine with dicarbonyl and related moieties in analogues of the C-terminal pentapeptide of cholecystokinin: CCK(2) agonists displaying a novel binding mode". Journal of Medicinal Chemistry 43 (20): 3614–23. October 2000. doi:10.1021/jm0000416. PMID 11020275.
- ↑ "Involvement of D2 dopamine receptors in the opposing effects of two CCK-B agonists in a spatial recognition memory task: role of the anterior nucleus accumbens". Psychopharmacology 153 (2): 170–9. January 2001. doi:10.1007/s002130000517. PMID 11205416.
- ↑ "How a single inversion of configuration leads to a reversal of the binding mode: proposal of a novel arrangement of CCK2 ligands in their receptor, and contribution to the development of peptidomimetic or non-peptide CCK2 ligands". European Journal of Medicinal Chemistry 38 (7–8): 671–86. 2003. doi:10.1016/S0223-5234(03)00112-0. PMID 12932898.
- ↑ "New CCK2 agonists confirming the heterogeneity of CCK2 receptors: characterisation of BBL454". Naunyn-Schmiedeberg's Archives of Pharmacology 370 (5): 404–13. November 2004. doi:10.1007/s00210-004-0969-7. PMID 15480577.
- ↑ "[Biological activity of cholecystokinin-(30-33) tetrapeptide analogs]" (in ru). Bioorganicheskaia Khimiia 31 (2): 130–9. 2005. PMID 15889786.
- ↑ "[Effect of a cholecystokinin tetrapeptide analogue on opioid reception under acute and chronic morphine administration]" (in ru). Bioorganicheskaia Khimiia 32 (3): 276–83. 2006. doi:10.1134/s106816200603006x. PMID 16808170.
- ↑ "Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors". Journal of Medicinal Chemistry 49 (10): 2868–75. May 2006. doi:10.1021/jm050921q. PMID 16686530.
- ↑ "Pharmacology of CCKRs and SAR studies of peptidic analog ligands". Current Topics in Medicinal Chemistry 7 (12): 1173–9. 2007. doi:10.2174/156802607780960447. PMID 17584139.
- ↑ "Strategies for design of non peptide CCK1R agonist/antagonist ligands". Current Topics in Medicinal Chemistry 7 (12): 1180–94. 2007. doi:10.2174/156802607780960537. PMID 17584140.
- ↑ "Strategies for the design of non-peptide CCK2 receptor agonist and antagonist ligand". Current Topics in Medicinal Chemistry 7 (12): 1195–204. 2007. doi:10.2174/156802607780960500. PMID 17584141.
 |
