Chemistry:Cadmium(I) tetrachloroaluminate
| |
| Names | |
|---|---|
| IUPAC name
dicadmium(2+) bis( tetrachoridoaluminate(1−))
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Cd 2[AlCl 4] 2 | |
| Molar mass | 562.4123 g/mol |
| Appearance | white crystal |
| Melting point | 227 ° (decomp)[clarification needed] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1027] TWA 0.005 mg/m3 (as Cd)[1] |
REL (Recommended)
|
Ca[1] |
IDLH (Immediate danger)
|
Ca [9 mg/m3 (as Cd)][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cadmium(I) tetrachloroaluminate is the inorganic compound with the formula Cd
2[AlCl
4]
2, a tetrachloroaluminate of cadmium(I). It was the first compound reported (1961) that contained cadmium in the +1 oxidation state and features a cadmium–cadmium bond.
Preparation and properties
Cd
2[AlCl
4]
2 was originally prepared by dissolving Cd metal in molten CdCl
2 followed by the addition of AlCl
3.[2]
- CdCl
2 + Cd → Cd
2Cl
2 - Cd
2Cl
2 + 2 AlCl
3 → Cd
2[AlCl
4]
2
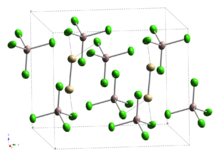
Subsequent studies of the Raman vibrational spectrum indicated the presence of a cadmium–cadmium bond,[3] which was confirmed by two separate X-ray diffraction studies of single crystals.[4][5] The compound can therefore be compared to mercury(I) (mercurous) compounds (such as mercury(I) chloride), which contain Hg2+
2. The Cd–Cd single bonds are part of ethane-like Cd
2Cl
6 units sharing vertices with AlCl
4 units, with a Cd–Cd bond length reported as 257.6 pm[4] or 256.1pm.[5]
Cd
2[AlCl
4]
2 is diamagnetic. It contains no unpaired electrons and reacts readily with water disproportionating to give Cd metal and Cd2+.

2Cl
6 unit
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0087.html.
- ↑ Corbett, J. D.; Burkhard, W. J.; Druding, L. F. (January 1961). "Stabilization of the Cadmium(I) Oxidation State. The System Cd-Cd12(AlCl4)2–Cd2(AlCl4)2". Journal of the American Chemical Society 83 (1): 76–80. doi:10.1021/ja01462a016.
- ↑ Corbett, J. D. (Aug 1962). "The Cadmium(I) Ion Cd2+2. Raman Spectrum and Relationship to Hg2+2". Inorganic Chemistry 1 (3): 700–703. doi:10.1021/ic50003a051.
- ↑ 4.0 4.1 Faggiani, R.; Ronald J. Gillespie; John E. Vekris (1986). "The cadmium(I) ion, Cd2+2; X-ray crystal structure of Cd2(AlCl4)2". Journal of the Chemical Society, Chemical Communications 1986 (7): 517–518. doi:10.1039/C39860000517.
- ↑ 5.0 5.1 Staffel, T.; Dr. Gerd Meyer (1987). "Synthesis and crystal structures of Cd[AlCl4]2 and Cd2[AlCl4]2". Zeitschrift für anorganische und allgemeine Chemie 548 (5): 45–54. doi:10.1002/zaac.19875480505.
 |

