Chemistry:Chavicine
From HandWiki
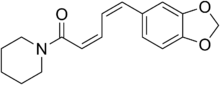
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z,4Z)-5-(2H-1,3-Benzodioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one | |
| Other names
(2Z,4Z)-5-(Benzo[d][1,3]dioxol-5-yl)-1-(piperidin-1-yl)penta-2,4-dien-1-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H19NO3 | |
| Molar mass | 285.343 g·mol−1 |
| Density | 1.211 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chavicine is a possibly pungent compound found in black pepper[1] and other species of the genus Piper. It is one of the four geometric isomers of piperine.
In light, especially ultra-violet light, chavicine is formed from its isomer piperine. Its flavor has been reported as flavorless[2][3] Chavicine will also re-isomerise back to piperine.[4]
See also
References
- ↑ Mărutoiu, Constantin; Gogoasa, Ioan; Oprean, Ioan; Mărutoiu, Olivia-Florena; Moise, Maria-Ioana; Tigae, Cristian; Rada, Maria (2006). "Separation and identification of piperine and chavicine in black pepper by TLC and GC-MS". Journal of Planar Chromatography: Modern TLC 19 (109): 250–252. doi:10.1556/JPC.19.2006.3.16.
- ↑ De Cleyn, R; Verzele, M (1972). "Constituents of peppers. I Qualitative Analysis of Piperine Isomers". Chromatografia 5: 346 - 350. https://www.chm.bris.ac.uk/sillymolecules/chavicine.pdf. Retrieved 26 September 2023.
- ↑ Tiwari, Anshuly; Mahadik, Kakasaheb R.; Gabhe, Satish Y. (2020). "Piperine: A comprehensive review of methods of isolation, purification, and biological properties". Medicine in Drug Discovery 7: 100027. http://doi.org/10.1016/j.medidd.2020.100027. Retrieved 26 September 2023.
- ↑ Kozukue, Nobuyuki; Park, Mal-Sun; others, and 5 (2007). "Kinetics of Light-Induced Cis−Trans Isomerization of Four Piperines and Their Levels in Ground Black Peppers as Determined by HPLC and LC/MS". J. Agric. Food Chem. 55 (17): 7131–7139. https://doi.org/10.1021/jf070831p. Retrieved 26 September 2023.
 |
