Chemistry:Chloropentammineplatinum chloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
Pentammineplatinum(IV) chloride, Pentammineplatinum chloride, Chloropentammineplatinum(IV) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Cl4H15N5Pt | |
| Molar mass | 422.04 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
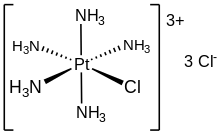
Chloropentammineplatinum chloride is the inorganic compound with the formula [PtCl(NH
3)
5]Cl
3. It is the chloride salt of the coordination complex [PtCl(NH
3)
5]+
. It is a white, water soluble solid.
The compound is prepared by treating potassium hexachloroplatinate with aqueous ammonia:[1]
- K
2PtCl
6 + 5 NH
3 → [PtCl(NH
3)
5]Cl
3 + 2 KCl
Related platinum(IV) ammines
The title complex is one of several platinum ammine complexes.
- Hexaammineplatinum(IV) chloride
- Trichlorotriammineplatinum(IV) chloride[2]
- cis-Tetrachlorodiammineplatinum(IV)[3]
- trans-Tetrachlorodiammineplatinum(IV) (RN 16986-23-5)[3]
References
- ↑ Curtis, Neville J.; Lawrance, Geoffrey A.; Sargeson, Alan M.; Johnson, Ronald C. (1986). "Pentaammineplatinum(IV) Complexes". Inorganic Syntheses. 24. pp. 277–279. doi:10.1002/9780470132555.ch74. ISBN 9780471834410.
- ↑ Gildengershel, Kh. I. (1966). "Synthesis of Cleve's salts [Pt(NH3)3Cl]Cl and [Pt(NH3)3Cl3]Cl". Zhurnal Prikladnoi Khimii 39: 223-5.
- ↑ 3.0 3.1 Kauffman, George B.; Slusarczuk, George; Kirschner, Stanley (1963). "cis ‐ and trans ‐Tetrachlorodiammineplatinum(IV)". Inorganic Syntheses. 7. pp. 236–238. doi:10.1002/9780470132388.ch62. ISBN 9780470131664.
 |

