Chemistry:Potassium hexachloroplatinate
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| Properties | |
| K2PtCl6 | |
| Molar mass | 485.99 g/mol |
| Appearance | orange to yellow solid |
| Density | 3.344 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) (decomposes) |
| 0.89 g/100ml (at 25 °C) [1] | |
Solubility product (Ksp)
|
7.48×10−6[2] |
| Hazards | |
| Safety data sheet | Oxford MSDS |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H301, H317, H318, H334 | |
| P261, P264, P270, P272, P280, P285, P301+310, P302+352, P304+341, P305+351+338, P310, P321, P330, P333+313, P342+311, P363, P405, P501 | |
| Flash point | 250 °C (482 °F; 523 K) |
| Related compounds | |
Other anions
|
Potassium tetrachloroplatinate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
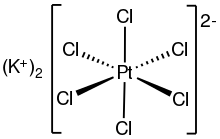
Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6. It is a yellow solid that is an example of a comparatively insoluble potassium salt. The salt features the hexachloroplatinate(IV) dianion, which has octahedral coordination geometry.
The precipitation of this compound from solutions of hexachloroplatinic acid was formerly used for the determination of potassium by gravimetric analysis.[4] It is also useful as an intermediate in the recovery of platinum from wastes.[5]
Reactions
Using salt metathesis reactions, potassium hexachloroplatinate is converted to a variety of quaternary ammonium and related lipophilic salts. These include tetrabutylammonium salt (NBu4)2PtCl6, which has been investigated as a catalyst.[6]
Reduction of potassium hexachloroplatinate with hydrazine dihydrochloride gives the corresponding tetrachloroplatinate salt.[7][8]
Potassium hexachloroplatinate reacts with aqueous ammonia to give chloropentammineplatinum chloride:[9]
- K
2PtCl
6 + 5 NH
3 → [PtCl(NH
3)
5]Cl
3 + 2 KCl
Safety
Dust containing potassium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."[10]
Related compounds
References
- ↑ Grinberg, A. A.; Sibirskaya, V. V. (1967). "Solubility of hexammine and hexahalo platinum(IV) complexes". Zhurnal Neorganicheskoi Khimii 12: 2069–2071.
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1-138-56163-2.
- ↑ "Potassium hexachloroplatinate(IV)" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/61856#section=Safety-and-Hazards.
- ↑ G. F. Smith; J. L. Gring (1933). "The Separation and Determination of the Alkali Metals Using Perchloric Acid. V. Perchloric Acid and Chloroplatinic Acid in the Determination of Small Amounts of Potassium in the Presence of Large Amounts of Sodium". J. Am. Chem. Soc. 55 (10): 3957–3961. doi:10.1021/ja01337a007.
- ↑ George B. Kauffman, Larry A. Teter "Recovery of Platinum from Laboratory Residues" Inorganic Syntheses, 1963, volume 7, pp. 232-236. doi:10.1002/9780470132388.ch61
- ↑ Iovel, I. G.; Goldberg, Y. S.; Shymanska, M. V.; Lukevics, E. (1987). "Quaternary Onium Hexachloroplatinates: Novel Hydrosilylation Catalysts". Organometallics 6 (7): 1410–1413. doi:10.1021/om00150a007.
- ↑ George B. Kauffman; Dwaine A. Cowan (1963). "Cis - and trans -Dichlorodiammineplatinum(II)". cis- and trans-Dichlorodiammine Platinum(II). Inorganic Syntheses. 7. pp. 239–245. doi:10.1002/9780470132388.ch63. ISBN 978-0-470-13238-8.
- ↑ Keller, R. N.; Moeller, T. (1963). "Potassium Tetrachloroplatinate(II)". Inorg. Synth. 7: 247–250. doi:10.1002/9780470132333.ch79.
- ↑ Curtis, Neville J.; Lawrance, Geoffrey A.; Sargeson, Alan M. (1986). "Pentaammineplatinum(IV) Complexes". Inorganic Syntheses 24: 277–279. doi:10.1002/9780470132555.ch74.
- ↑ Renner, Hermann; Schlamp, Günther; Kleinwächter, Ingo; Drost, Ernst; Lüschow, Hans Martin; Tews, Peter; Panster, Peter; Diehl, Manfred et al. (2001). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_075. ISBN 3-527-30673-0.
 |
