Chemistry:Choline chloride
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxy-N,N,N-trimethylethan-1-aminium chloride | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
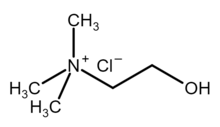
| [(CH 3) 3NCH 2CH 2OH]+ Cl− | |
| Molar mass | 139.62 g·mol−1 |
| Appearance | White hygroscopic crystals |
| Melting point | 302 °C (576 °F; 575 K) (decomposes) |
| very soluble (>650 g/L)[1] | |
| Hazards | |
| Main hazards | Corrosive |
| Safety data sheet | External MSDS |
| GHS Signal word | Danger |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Choline chloride is an organic compound with the formula [(CH
3)
3NCH
2CH
2OH]+
Cl−
. It is a quaternary ammonium salt, consisting of choline cations ([(CH
3)
3NCH
2CH
2OH]+
) and chloride anions (Cl−
). It is a bifunctional compound, meaning, it contains both a quaternary ammonium functional group and a hydroxyl functional group. The cation of this salt, choline, occurs in nature in living beings.[2] Choline chloride is a white, water-soluble salt used mainly in animal feed.[3]
Synthesis
In the laboratory, choline can be prepared by methylation of dimethylethanolamine with methyl chloride.
Choline chloride is mass-produced with world production estimated at 160 000 tons in 1999.[3] Industrially, it is produced by the reaction of ethylene oxide, hydrogen chloride, and trimethylamine,[4] or from the pre-formed salt:[5]
Choline chloride can also be made by treating trimethylamine with 2-chloroethanol.[6]
- (CH
3)
3N + ClCH
2CH
2OH → [(CH
3)
3NCH
2CH
2OH]+
Cl−
Applications
It is an important additive in feed especially for chickens where it accelerates growth. It forms a deep eutectic solvent with urea, ethylene glycol, glycerol, and many other compounds.
It is also used as a clay control additive in fluids used for hydraulic fracturing.[7]
Related salts
Other commercial choline salts are choline hydroxide and choline bitartrate. In foodstuffs, the compound is often present as phosphatidylcholine.
References
- ↑ "Chemical Safety Information from Intergovernmental Organizations - Choline Chloride". http://www.inchem.org/documents/sids/sids/67481.pdf.
- ↑ "Choline". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. February 2015. http://lpi.oregonstate.edu/mic/other-nutrients/choline.
- ↑ 3.0 3.1 Matthias Frauenkron; Johann-Peter Melder; Günther Ruider; Roland Rossbacher; Hartmut Höke (2002). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001. ISBN 3527306730.
- ↑ |title=Johnson Matthey Process Technology - Choline chloride licensed process
- ↑ "Choline chloride". Screening Information Data Set (SIDS) for High Production Volume Chemicals. IPCS INCHEM. http://www.inchem.org/documents/sids/sids/67481.pdf.
- ↑ Kirk-Othmer encyclopedia of chemical technology. 6 (4th ed.). John Wiley & Sons. 2000. pp. 100–102. ISBN 9780471484943.
- ↑ "What Chemicals Are Used". http://fracfocus.org/chemical-use/what-chemicals-are-used. Retrieved 19 September 2014.
 |

