Chemistry:Chromite (compound)
From HandWiki
In chemistry the term chromite has been used in two contexts. Under IUPAC naming conventions, chromate(III) is preferred to chromite.[citation needed]
- For compounds containing an oxyanion of chromium in oxidation state of +3
- For other compounds of chromium(III) as a means of distinguishing a chemical species such as hexacyanochromite(III). [Cr(CN)6]3− from an analogous compound in which chromium is a different oxidation state.
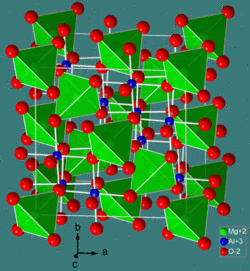
The mineral chromite is an iron chromium oxide with empirical formula FeCr2O4. Structurally, it belongs to the spinel group. Magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4);.[1] Zincochromite is another example. The crystal structure of the acid, HCrO2 has been determined by neutron diffraction.[2]
Chromites may be formed by reaction of chromium(III) oxide with a metal oxide:[3]
- Cr2O3 + MgO → MgCr2O4
References
- ↑ http://www.mindat.org/min-8675.html Mindat
- ↑ Hamilton, W. C.; Ibers, J. A. (1963). "Structures of HCrO2 and DCrO2". Acta Crystallographica 16 (12): 1209–1212. doi:10.1107/S0365110X63003182.
- ↑ Kunnmann, W. (1973). "Magnesium Chromite: (Magnesium Chromium (III) Oxide)". Inorganic Syntheses 14: 134. doi:10.1002/9780470132456.ch26.
 |