Chemistry:Cilofexor
From HandWiki
Short description: Drug in clinical trials
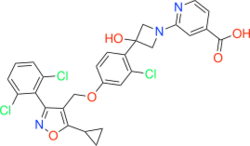 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C28H22Cl3N3O5 |
| Molar mass | 586.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cilofexor (also known as GS-9674) is a nonsteroidal farnesoid X receptor (FXR) agonist in clinical trials for the treatment of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH),[1][2][3] and primary sclerosing cholangitis (PSC).[4][5] It is being investigated for use alone or in combination with firsocostat, selonsertib,[1] or semaglutide.[2][6] In rat models[3] and human clinical trials[7] of NASH it has been shown to reduce fibrosis and steatosis, and in human clinical trials of PSC it improved cholestasis and reduced markers of liver injury.[4]
It is being developed by the pharmaceutical company Gilead Sciences.[8][6]
References
- ↑ 1.0 1.1 Clinical trial number NCT02781584 for "Safety, Tolerability, and Efficacy of Selonsertib, Firsocostat, and Cilofexor in Adults With Nonalcoholic Steatohepatitis (NASH)" at ClinicalTrials.gov
- ↑ 2.0 2.1 Clinical trial number NCT04971785 for "Study Evaluating the Safety and Efficacy of Semaglutide, and the Fixed-Dose Combination of Cilofexor and Firsocostat, Alone and in Combination, in Participants With Compensated Cirrhosis (F4) Due to Nonalcoholic Steatohepatitis (NASH)" at ClinicalTrials.gov
- ↑ 3.0 3.1 "The Non-Steroidal FXR Agonist Cilofexor Improves Portal Hypertension and Reduces Hepatic Fibrosis in a Rat NASH Model". Biomedicines 9 (1): 60. 9 Jan 2021. doi:10.3390/biomedicines9010060. PMID 33435509.
- ↑ 4.0 4.1 "The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis". Hepatology 70 (3): 788–801. 19 January 2019. doi:10.1002/hep.30509. PMID 30661255.
- ↑ Clinical trial number NCT03890120 for "Safety, Tolerability, and Efficacy of Cilofexor in Non-Cirrhotic Adults With Primary Sclerosing Cholangitis (PRIMIS)" at ClinicalTrials.gov
- ↑ 6.0 6.1 "Gilead and Novo Nordisk Expand NASH Clinical Collaboration" (Press release). Gilead. 18 March 2021. Archived from the original on 2022-01-20.
- ↑ "Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial". Hepatology 72 (1): 58–71. 9 Jan 2021. doi:10.1002/hep.31205. PMID 32115759.
- ↑ "Gilead Announces Topline Results From Phase 2 ATLAS Study in Patients With Bridging Fibrosis (F3) and Compensated Cirrhosis (F4) Due to Nonalcoholic Steatohepatitis (NASH)" (Press release). Gilead. 18 March 2021. Archived from the original on 2022-01-21.
 |
