Biology:Semaglutide
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɛmˈæɡlʊtaɪd/ sem-AG-luu-tyde or /ˌsɛməˈɡluːtaɪd/ SEM-ə-GLOO-tyde |
| Trade names | Ozempic, Rybelsus, Wegovy, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618008 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Subcutaneous, oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89% |
| Metabolism | Proteolysis |
| Elimination half-life | 7 days |
| Duration of action | 63.6 h |
| Excretion | Urine and feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
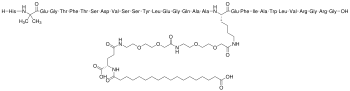
| Formula | C187H291N45O59 |
| Molar mass | 4113.641 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Semaglutide is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management.[17][18][19] A once-a-week regimen was trialed in a 12-week study by Novo Nordisk in 2012, and it was approved for use in the US in 2017. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain.[20][21] It can be administered by subcutaneous injection or taken orally.[22][11][12][13] It is sold under the brand names Ozempic (injectable)[11] and Rybelsus (pill)[12] for diabetes, and under the brand name Wegovy for weight loss.[13]
Semaglutide is a glucagon-like peptide-1 receptor agonist.[11][12][13] The most common side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation.[11][14][15][16][23]
In 2021, semaglutide was the 90th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[24][25]
Medical uses
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.[11][12]
The higher-dose formulation of semaglutide is indicated as an adjunct to diet and exercise for long-term weight management in adults with obesity (initial body mass index (BMI) ≥ 30 kg/m2) or who are overweight (initial BMI ≥ 27 kg/m2) and have at least one weight-related comorbidity.[13][16][26] Oral semaglutide is marketed in three strengths: 3 mg, 7 mg and 14 mg. The subcutaneous injection of semaglutide is available in four strengths: 0.25 mg, 0.5 mg, 1 mg, 1.7 mg, 2 mg, and 2.4 mg.[13]
Eating disorders
Semaglutide and similar drugs, such as dulaglutide and liraglutide, have been used to treat binge eating disorder (BED), as they can successfully minimize obsessive thoughts about food and binging urges.[27][28][29][30] Some users of these drugs have reported significant reduction in what is colloquially known as "food noise" (constant, unstoppable thoughts about eating despite not being physically hungry), which can be a factor of BED.[31][32] As of January 2024, more research is needed.[33]
Adverse effects
Possible side effects include nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, indigestion/heartburn, dizziness, abdominal distension, belching, hypoglycemia (low blood glucose) in people with type 2 diabetes, flatulence, gastroenteritis, and gastroesophageal reflux disease (GERD).[13] It can also cause pancreatitis, gastroparesis, and bowel obstruction.[34]
The US FDA label for semaglutide contains a boxed warning for thyroid C-cell tumors in rodents.[11][12][13][23] It is unknown whether semaglutide causes thyroid C-cell tumors, including medullary thyroid carcinoma, in humans.[11][12][13][23][35]
Contraindications
Data from rodent studies of GLP-1-mediated thyroid C-cell hyperplasia[36] indicates that use is contraindicated in people with a personal or family history of medullary thyroid carcinoma or with multiple endocrine neoplasia type 2.[12][11]
Mechanism of action
Semaglutide is a glucagon-like peptide-1 receptor agonist.[11][12][13] The drug decreases blood sugar levels. The decrease is theorized to be caused by the mimicking of the incretin glucagon-like peptide-1 (GLP-1).[37] It also appears to enhance growth of pancreatic beta cells, which are responsible for insulin production and release.[21][38] Additionally, it inhibits the production of glucagon, the hormone that increases glycogenolysis (release of stored carbohydrate from the liver) and gluconeogenesis (synthesis of new glucose). It reduces food intake by lowering appetite and slowing down digestion in the stomach,[20] helping reduce body fat.[39][40]
Structure and pharmacology
Semaglutide is chemically similar to human GLP-1.[41] The first six amino acids of GLP-1 are missing.[41] Substitutions are made at GLP positions 8 and 34 (semaglutide positions 2 and 28), where alanine and lysine are replaced by 2-aminoisobutyric acid and arginine, respectively.[41] The substitution of the alanine prevents chemical breakdown by dipeptidyl peptidase-4.[42] The lysine at GLP position 26 (semaglutide position 20) has a long chain attached, ending with a chain of 17 carbon atoms and a carboxyl group.[42] This increases the drug's binding to blood protein (albumin), which enables longer presence in the blood circulation.[42]
Semaglutide's half-life in the blood is about seven days (165–184 hours).[21][43] It can be administered by subcutaneous injection or taken orally (by mouth).[11][12][13]
History
In June 2008, a phase II clinical trial began studying semaglutide.[44]
In 2012, a team of researchers at Novo Nordisk developed semaglutide[45] for a once-weekly diabetes therapy as a longer-acting alternative to liraglutide.[46] It was given the brand name Ozempic. Clinical trials started in January 2016 and ended in May 2017.[17][47]
In March 2021, in a phase III randomized, double-blind, trial, 1,961 adults with a body mass index of 30 or greater were assigned in a 2:1 ratio to a treatment with once-weekly subcutaneous semaglutide or placebo, plus lifestyle intervention. The trials occurred at 129 sites in 16 countries in Asia, Europe, North America, and South America. The mean percentage change in body weight at week 68 was −14.9% in the semaglutide group vs −2.4% with placebo, for an estimated treatment difference of −12.4 percentage points (95% CI, −13.4 to −11.5).[48][49][50][51]
A 2022 review of anti-obesity treatments found that semaglutide as well as tirzepatide (which has an overlapping mechanism of action) were more promising than previous anti-obesity drugs, although less effective than bariatric surgery.[52]
The US Food and Drug Administration (FDA) approved semaglutide based on evidence from seven clinical trials of 4087 participants with type 2 diabetes.[23] The trials were conducted at 536 sites in 33 countries, including Canada, Mexico, Russian Federation, Ukraine, Turkey, India, South Africa, Japan, Hong Kong, multiple European countries, Argentina, and the United States.[23] In two of these trials (NCT #02054897 and NCT#02305381), participants were randomly assigned to receive either semaglutide or placebo injection weekly.[23] Neither the participant nor the health care provider knew which treatment was being given until after the trials were completed.[23] Treatment was given for 30 weeks.[23] In the other five trials (NCT #01930188, 01885208, 02128932, 02207374, 02254291), participants were randomly assigned to receive either semaglutide or another antidiabetic medication, and the participant and provider knew which medication was being given in four trials.[23] Treatment was given for 30 weeks or 56 weeks.[23]
In each trial, HbA1c was measured from the start of the trial to the end of the trial and compared between the semaglutide group and the other groups.[23]
The FDA also considered data from one separate trial (NCT #01720446) of 3297 participants with type 2 diabetes who were at high risk for cardiovascular events.[23] This trial was conducted in 20 countries in Europe, Russian Federation, Turkey, Brazil, Israel, Malaysia, Brazil, Mexico, Thailand, Taiwan, Canada, and the United States.[23] The participants were randomly assigned to receive semaglutide or placebo.[23] Neither the participant nor the health care provider knew which treatment was being given.[23] Treatment was given for 104 weeks (2 years), and the occurrence of cardiovascular events, including heart attacks, strokes and hospitalization due to unstable angina (near heart attack) were recorded and compared in the two groups of participants.[23]
Society and culture
Legal status
In December 2016, the US FDA New Drug Application (NDA) was filed, and in October 2017, the FDA Advisory Committee approved it unanimously.[53]
In December 2017, the injectable version with the brand name Ozempic was approved for use by people with diabetes in the United States,[54][55] and, in January 2018, in Canada.[56]
In February 2018, authorization was granted in the European Union,[14][57] in March 2018 in Japan,[58] and in August 2019 in Australia.[1][3]
In September 2019, a version that can be taken orally (Rybelsus) was approved for medical use in the United States,[59][60] and in the European Union in April 2020.[15]
In June 2021, a higher-dose version for injectable use sold under the brand name Wegovy was approved by the US Food and Drug Administration (FDA) as an anti-obesity medication for long-term weight management in adults.[13] In November 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended to grant a marketing authorization for Wegovy[61] to Novo Nordisk A/S.[61] In January 2022, Wegovy was approved for medical use in the European Union.[16][62]
In January 2023, the label for Rybelsus was updated to reflect that it can be used as a first-line treatment for adults with type 2 diabetes.[63]
Gray market sellers offer unauthorized products claimed to be semaglutide online. This practice is illegal in the United States, but some buyers turn to unauthorized retailers due to being denied insurance coverage and not being able to afford the name brand drug.[64][65][66][67][68]
In October 2023, Belgium announced it was considering a temporary ban on Ozempic for use as a weight loss medication amid a sharp increase in demand leading to a shortage of the drug, which was expected to last into mid-2024. The government had previously advised medical professionals to prescribe the drug only to diabetics. A similar order had been issued by the government of the United Kingdom earlier that month, additionally prohibiting new prescriptions for type 2 diabetics.[69]
Generics
The patent was scheduled to expire in 2026, but a Chinese court ruled in 2022 that all patents on semaglutide were invalid. Novo Nordisk appealed the ruling.[70]
In Brazil, the Supreme Court refused to extend semaglutide's patent protection, which expires in 2026.[71]
Economics
"Ozempic, the semaglutide injection used for T2D treatment, has a list price of $936 in the United States and $169 in Japan. Prices were $147 in Canada, $144 in Switzerland, $103 in Germany and Netherlands, $96 in Sweden, $93 in the United Kingdom, and $87 in Australia. France had the lowest price at $83." (August 21, 2023)[72][73]
In the US, Wegovy has a list price of $1,349.02 per month as of 2022 suggesting that because of the high costs many people "who could most benefit from weight loss may be unable to afford such expensive drugs".[74] High costs of Ozempic prompted some insurance companies to investigate and refuse to cover patients with what the companies considered was insufficient evidence to support a diabetes diagnosis, alleging off-label prescribing for weight loss.[75]
In the UK, semaglutide is available on NHS prescription for diabetes at nominal or no cost to patients.[76] It is also available for obesity, limited to treatment for two years.[77]
High demand caused worldwide supply shortages of semaglutide in 2023;[75] new UK prescriptions were not issued during the shortage.
By 2023, Novo Nordisk had become the most valuable corporation in the European Union, worth more than US$500 billion, and accounting for almost all of recent economic growth in Denmark.[78]
Counterfeits
In October 2023, there were reports of counterfeit Ozempic pens being sold in Europe.[79] The pens possibly contained insulin, and led to several people being hospitalised with hypoglycaemia and seizures.[80][81][82] In December 2023, the FDA issued a warning about counterfeit Ozempic in the United States.[83]
Some counterfeits have been found to contain salts of semaglutide including the sodium and the acetate in an attempt to avoid the patent of the base semaglutide product and are not considered safe or effective by the US Food and Drug Administration (FDA).[84]
Research
A 2014 meta-analysis found that semaglutide may be effective in lowering liver enzymes (transaminitis) and improving certain radiologically observed features of metabolic dysfunction–associated steatotic liver disease.[85] French national health care insurance system database had previously suggested 1–3 years use of glucagon like peptide-1 receptor agonists like exenatide, liraglutide and dulaglutide may be linked with increased occurrence of thyroid cancer. Semaglutide belongs to the same family of medicine. A meta-analysis involving data from 37 randomized controlled trials and 19 real world studies (46,719 patients) showed that semaglutide use over 18 months was not associated with increased risks of any cancer, supported by a high grade of evidence.[86]
A study funded by Eli Lilly, producer of tirzepatide, in 2021 found that semaglutide, when used once weekly as add-on therapy to metformin in people with type 2 diabetes, was found to be inferior to tirzepatide (sold under the brand name Mounjaro) in both endpoints of reduction in glycohemoglobin, A1C, and body weight, with a roughly similar safety profile. The same study pointed out that semaglutide was found to cause less serious adverse events compared to tirzepatide.[87]
In July 2023, the Icelandic Medicines Agency reported two cases of suicidal thoughts and one case of self-injury of users of the injection, prompting a safety assessment of Ozempic,[88] Wegovy, Saxenda, and similar drugs.[89] In January 2024, a preliminary review conducted by the FDA confirmed no evidence had been found to suggest that the medicine causes suicidal thoughts or actions.[90][91]
References
- ↑ 1.0 1.1 1.2 "AusPAR: Semaglutide". 2 December 2020. https://www.tga.gov.au/auspar/auspar-semaglutide.
- ↑ 2.0 2.1 "Rybelsus APMDS". 22 February 2022. https://www.tga.gov.au/resources/auspmd/rybelsus.
- ↑ 3.0 3.1 "Summary for ARTG Entry:315107 Ozempic 1 mg semaglutide (rys) 1.34 mg/mL solution for injection pre-filled pen". https://tga-search.clients.funnelback.com/s/search.html?collection=tga-artg&profile=record&meta_i=315107.
- ↑ "Summary for ARTG Entry: 346198 Rybelsus semaglutide 3 mg tablet blister pack". https://tga-search.clients.funnelback.com/s/search.html?collection=tga-artg&profile=record&meta_i=346198.
- ↑ "Wegovy (Novo Nordisk Pharmaceuticals Pty Ltd)". 7 October 2022. https://www.tga.gov.au/resources/prescription-medicines-registrations/wegovy-novo-nordisk-pharmaceuticals-pty-ltd.
- ↑ Product Monograph Including Patient Medication Information – Ozempic semaglutide injection (Report). Novo-Nordisk Canada. 21 August 2020. https://pdf.hres.ca/dpd_pm/00058023.PDF. Retrieved 6 June 2021.
- ↑ Product Monograph Including Patient Medication Information – Rybelsus semaglutide tablets (Report). Novo-Nordisk Canada. 30 March 2020. https://pdf.hres.ca/dpd_pm/00055582.PDF. Retrieved 6 June 2021.
- ↑ "Regulatory Decision Summary – Rybelsus". 23 October 2014. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?linkID=RDS00625.
- ↑ "Ozempic 0.25 mg solution for injection in pre-filled pen – Summary of Product Characteristics (SmPC)". 9 April 2021. https://www.medicines.org.uk/emc/product/9748/smpc.
- ↑ "Rybelsus – Summary of Product Characteristics (SmPC)". 25 November 2020. https://www.medicines.org.uk/emc/product/11507/smpc.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 "Ozempic- semaglutide injection, solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=adec4fd2-6858-4c99-91d4-531f5f2a2d79.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 "Rybelsus- oral semaglutide tablet". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=27f15fac-7d98-4114-a2ec-92494a91da98.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 "Wegovy- semaglutide injection, solution". 4 June 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee06186f-2aa3-4990-a760-757579d8f77b.
- ↑ 14.0 14.1 14.2 "Ozempic EPAR". European Medicines Agency (EMA). 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ozempic.
- ↑ 15.0 15.1 15.2 "Rybelsus EPAR". 29 January 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus.
- ↑ 16.0 16.1 16.2 16.3 "Wegovy EPAR". 11 November 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy.
- ↑ 17.0 17.1 "Wegovy (semaglutide): a new weight loss drug for chronic weight management". Journal of Investigative Medicine 70 (1): 5–13. January 2022. doi:10.1136/jim-2021-001952. PMID 34706925.
- ↑ "Clinical review of subcutaneous semaglutide for obesity". Journal of Clinical Pharmacy and Therapeutics 47 (2): 184–193. February 2022. doi:10.1111/jcpt.13574. PMID 34964141.
- ↑ "Efficacy and safety of semaglutide for weight management: evidence from the STEP program". Postgraduate Medicine 134 (sup1): 5–17. January 2022. doi:10.1080/00325481.2022.2147326. PMID 36691309.
- ↑ 20.0 20.1 "Semaglutide in type 2 diabetes - is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)?". Expert Opinion on Drug Metabolism & Toxicology 14 (3): 371–377. March 2018. doi:10.1080/17425255.2018.1441286. PMID 29439603. https://eprints.qut.edu.au/121664/2/121664.pdf. Retrieved 12 December 2019.
- ↑ 21.0 21.1 21.2 "Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes". Canadian Journal of Diabetes 43 (2): 136–145. March 2019. doi:10.1016/j.jcjd.2018.05.008. PMID 30195966.
- ↑ "Oral Semaglutide". Clinical Diabetes 38 (1): 109–111. January 2020. doi:10.2337/cd19-0079. PMID 31975761.
- ↑ 23.00 23.01 23.02 23.03 23.04 23.05 23.06 23.07 23.08 23.09 23.10 23.11 23.12 23.13 23.14 23.15 "Ozempic Drug Trial Snapshot". 5 December 2017. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-ozempic.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Semaglutide – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Semaglutide.
- ↑ "FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014". U.S. Food and Drug Administration (FDA) (Press release). 4 June 2021. Archived from the original on 4 June 2021. Retrieved 5 June 2021.
- ↑ Hayashi, Daisuke; Edwards, Caitlyn; Emond, Jennifer A.; Gilbert-Diamond, Diane; Butt, Melissa; Andrea, Rigby; Masterson, Travis D. (November 17, 2023). Trakada, Georgia. ed. "What Is Food Noise? A Conceptual Model of Food Cue Reactivity". Nutrients 15 (22): 4809. doi:10.3390/nu15224809. PMID 38004203.
- ↑ Da Porto, Andrea; Casarsa, Viviana; Colussi, Gianluca; Catena, Cristiana; Cavarape, Alessandro; Sechi, Leonardo (August 2020). "Dulaglutide reduces binge episodes in type 2 diabetic patients with binge eating disorder: A pilot study". Diabetology & Metabolic Syndrome 14 (4): 289–292. doi:10.1016/j.dsx.2020.03.009. PMID 32289741. https://www.sciencedirect.com/science/article/abs/pii/S1871402120300497. Retrieved January 13, 2024.
- ↑ Robert, Sarah Anne; Rohana, Abdul Ghani; Shah, Shamsul Azhar; Chinna, Karuthan; Wan Mohamud, Wan Naizamoon; Kamaruddin, Nor Azmi (April 11, 2015). "Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide – A pilot study". Obesity Research & Clinical Practice 9 (3): 301–304. doi:10.1016/j.orcp.2015.03.005. PMID 25870084. https://www.sciencedirect.com/science/article/abs/pii/S1871403X1500037X. Retrieved January 13, 2014.
- ↑ Järvinen, Anna; Laine, Merja K.; Tikkanen, Roope; Castrén, Maija L. (2019). "Beneficial Effects of GLP-1 Agonist in a Male With Compulsive Food-Related Behavior Associated With Autism". Frontiers in Psychiatry 10: 97. doi:10.3389/fpsyt.2019.00097. PMID 30881319.
- ↑ Kuhn, Casey (September 25, 2023). "Patients say drugs like Ozempic help with 'food noise.' Here's what that means". PBS. https://www.pbs.org/newshour/health/patients-say-drugs-like-ozempic-help-with-food-noise-heres-what-that-means.
- ↑ Blum, Dani (June 21, 2023). "People on Drugs Like Ozempic Say Their 'Food Noise' Has Disappeared". The New York Times. https://www.nytimes.com/2023/06/21/well/eat/ozempic-food-noise.html.
- ↑ Richards, Jesse; Bang, Neha; Ratliff, Erin L.; Paszkowiak, Maria A.; Khorgami, Zhamak; Khalsa, Sahib S.; Simmons, W. Kyle (2023). "Successful treatment of binge eating disorder with the GLP-1 agonist semaglutide: A retrospective cohort study". Obesity Pillars 7. doi:10.1016/j.obpill.2023.100080. PMID 37990682.
- ↑ "Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss". The Journal of the American Medical Association 330 (18): 1795–1797. October 2023. doi:10.1001/jama.2023.19574. PMID 37796527.
- ↑ "The Thyroid Pathologist Meets Therapeutic Pharmacology". Endocrine Pathology 34 (1): 48–56. March 2023. doi:10.1007/s12022-023-09749-1. PMID 36723855.
- ↑ "Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation". Endocrinology 151 (4): 1473–1486. April 2010. doi:10.1210/en.2009-1272. PMID 20203154.
- ↑ "Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes". The New England Journal of Medicine 375 (19): 1834–1844. November 2016. doi:10.1056/NEJMoa1607141. PMID 27633186.
- ↑ "Glucagon-like Peptide-1 Receptor Signaling Modulates β Cell Apoptosis". The Journal of Biological Chemistry 278 (1): 471–478. 2003. doi:10.1074/jbc.M209423200. PMID 12409292.
- ↑ "Semaglutide: First Global Approval". Drugs 78 (2): 275–284. February 2018. doi:10.1007/s40265-018-0871-0. PMID 29363040.
- ↑ "Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity". Diabetes, Obesity & Metabolism 19 (9): 1242–1251. September 2017. doi:10.1111/dom.12932. PMID 28266779.
- ↑ 41.0 41.1 41.2 "Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide". Journal of Medicinal Chemistry 58 (18): 7370–80. September 2015. doi:10.1021/acs.jmedchem.5b00726. PMID 26308095.
- ↑ 42.0 42.1 42.2 "The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates". Diabetes 63 (7): 2486–97. July 2014. doi:10.2337/db13-1087. PMID 24608440.
- ↑ "Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel". Journal of Clinical Pharmacology 55 (5): 497–504. May 2015. doi:10.1002/jcph.443. PMID 25475122.
- ↑ Novo Nordisk A/S (13 June 2008). A Randomised Controlled Clinical Trial in Type 2 Diabetes Comparing Semaglutide to Placebo and Liraglutide (Report). NCT00696657. https://clinicaltrials.gov/ct2/show/NCT00696657. Retrieved 24 March 2023.
- ↑ "Abstracts of the 48th EASD (European Association for the Study of Diabetes) Annual Meeting of the European Association for the Study of Diabetes. October 1–5, 2012. Berlin, Germany". Diabetologia 55 (S1): S7–537. October 2012. doi:10.1007/s00125-012-2688-9. PMID 22918257.
- ↑ "Once-weekly glucagon-like peptide 1 receptor agonists". The Journal of the Pakistan Medical Association 65 (7): 796–8. July 2015. PMID 26160096. https://jpma.org.pk/article-details/7416?article_id=7416. Retrieved 10 April 2022.
- ↑ Novo Nordisk A/S (2 October 2019). "Efficacy and Safety of Semaglutide Versus Dulaglutide as add-on to Metformin in Subjects With Type 2 Diabetes". ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02648204. Retrieved 3 March 2023.
- ↑ "Once-Weekly Semaglutide in Adults with Overweight or Obesity". The New England Journal of Medicine 384 (11): 989–1002. March 2021. doi:10.1056/NEJMoa2032183. PMID 33567185.
- ↑ "What Is Ozempic and Why Is It Getting So Much Attention?". The New York Times. 22 November 2022. ISSN 0362-4331. https://www.nytimes.com/2022/11/22/well/ozempic-diabetes-weight-loss.html.
- ↑ "Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial". Nature Medicine 28 (10): 2083–2091. October 2022. doi:10.1038/s41591-022-02026-4. PMID 36216945.
- ↑ "WHO advisers to consider whether obesity medication should be added to Essential Medicines List". 29 March 2023. https://www.cnn.com/2023/03/29/health/who-essential-medicines-liraglutide/index.html.
- ↑ "Anti-obesity drug discovery: advances and challenges". Nature Reviews. Drug Discovery 21 (3): 201–223. March 2022. doi:10.1038/s41573-021-00337-8. PMID 34815532.
- ↑ "Development Status and FDA Approval Process for semaglutide". Drugs.com. 2017. https://www.drugs.com/history/semaglutide.html.
- ↑ "Drug Approval Package: Ozempic (semaglutide) Injection". U.S. Food and Drug Administration (FDA). 16 January 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209637Orig1s000TOC.cfm.
- ↑ "Ozempic (semaglutide) approved in the US". Novo Nordisk (Press release). 5 December 2017. Archived from the original on 5 June 2021. Retrieved 5 June 2021.
- ↑ "Regulatory Decision Summary – Ozempic". Health Canada. 23 October 2014. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00317.
- ↑ "Novo Nordisk A/S: Ozempic (semaglutide) approved in the EU for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 9 February 2018. Archived from the original on 2 April 2019. Retrieved 19 August 2018 – via GlobeNewswire.
- ↑ "Ozempic approved in Japan for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 23 March 2018. Archived from the original on 2 April 2019. Retrieved 2 April 2019 – via GlobeNewswire.
- ↑ "Drug Approval Package: Rybelsus". U.S. Food and Drug Administration (FDA). 10 June 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/213051Orig1s000TOC.cfm.
- ↑ "FDA approves first oral GLP-1 treatment for type 2 diabetes" (Press release). FDA. 20 September 2019. Archived from the original on 23 September 2019. Retrieved 20 September 2019.
- ↑ 61.0 61.1 "Wegovy : Pending EC decision". 11 November 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/wegovy. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Wegovy Product information". https://ec.europa.eu/health/documents/community-register/html/h1608.htm.
- ↑ "Novo Nordisk announces FDA approval of label update for Rybelsus (semaglutide) allowing use as a first-line option for adults with type 2 diabetes" (Press release). Novo Nordisk. 12 January 2023. Archived from the original on 16 January 2023. Retrieved 16 January 2023 – via PR Newswire.
- ↑ "Woman says she got less expensive drug for weight loss after being denied by insurance". ABC News. https://abcnews.go.com/GMA/Wellness/high-cost-drugs-weight-loss-ozempic-mounjaro-users/story?id=99424157.
- ↑ "Safety worries over copycat versions of Ozempic and Wegovy prompt state crackdowns". NBC News. 3 May 2023. https://www.nbcnews.com/health/health-news/ozempic-wegovy-weight-loss-compounded-crackdowns-rcna82405.
- ↑ "The FDA Warned Ozempic Users. They Don't Give a F-ck". Rolling Stone. 8 June 2023. https://www.rollingstone.com/culture/culture-features/ozempic-semaglutide-fda-warning-compound-drug-1234766348/. Retrieved 26 September 2023.
- ↑ "Inside the gold rush to sell cheaper imitations of Ozempic". Washington Post. 19 September 2023. https://www.washingtonpost.com/business/2023/09/19/ozempic-semaglutide-compounding-pharmacies/.
- ↑ "The high price of Ozempic is pushing many to unregulated, copycat drugs for weight loss". NBC News. 19 March 2023. https://www.nbcnews.com/health/health-news/ozempic-wegovy-semaglutide-compounding-weight-loss-safe-rcna72990.
- ↑ Strauss, Marine (24 October 2023). "Belgium plans temporary ban on use of Ozempic for weight loss". Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/belgium-plans-temporary-ban-use-ozempic-weight-loss-2023-10-24/.
- ↑ He, Laura (7 June 2023). "Ozempic is taking China by storm. Drugmakers are scrambling to boost supplies | CNN Business". CNN. https://www.cnn.com/2023/06/06/business/china-ozempic-drug-shortage-intl-hnk/index.html.
- ↑ Masters, William (9 May 2023). "Brazilian federal court denies semaglutide patent extension". Pharmaceutical Technology. https://www.pharmaceutical-technology.com/pricing-and-market-access/brazilian-federal-court-semaglutide-patent-extension/?cf-view.
- ↑ "U.S. Leads the world in list prices for diabetes, antiobesity meds". 21 August 2023. https://www.medicaleconomics.com/view/u-s-leads-the-world-in-list-prices-for-diabetes-antiobesity-meds.
- ↑ "Charted: The cost of weight-loss drugs in the US vs. Other countries". https://www.advisory.com/daily-briefing/2023/08/21/weight-loss-drug-cost.
- ↑ "Patients Taking Experimental Obesity Drug Lost More Than 50 Pounds, Maker Claims". The New York Times. 28 April 2022. https://www.nytimes.com/2022/04/28/health/obesity-drug-eli-lilly-tirzepatide-wegovy.html.
- ↑ 75.0 75.1 "Insurers clamping down on doctors who prescribe Ozempic for weight loss". 12 June 2023. https://www.washingtonpost.com/business/2023/06/11/weight-loss-ozempic-wegovy-insurance/.
- ↑ "NICE recommended weight-loss drug to be made available in specialist NHS services". National Institute for Health and Care Excellence (NICE). 8 March 2023. https://www.nice.org.uk/news/article/nice-recommended-weight-loss-drug-to-be-made-available-in-specialist-nhs-services.
- ↑ "I lost 40 pounds on Ozempic. But I'm left with even more questions". The Washington Post. 6 June 2023. https://www.washingtonpost.com/opinions/2023/06/06/ozempic-weight-loss-ruth-marcus/.
- ↑ Verrender, Ian (19 December 2023). "How Ozempic could affect the health of the global economy in more ways than one". ABC News. https://www.abc.net.au/news/2023-12-19/ozempic-obesity-diabetes-health-challenges-impact-global-economy/103244056.
- ↑ "EMA alerts EU patients and healthcare professionals to reports of falsified Ozempic pens". European Medicines Agency (Press release). 18 October 2023. Archived from the original on 4 January 2024. Retrieved 6 January 2024.
- ↑ "Ozempic (semaglutide) and Saxenda (liraglutide): vigilance required due to potentially harmful falsified products". 23 November 2023. https://www.gov.uk/drug-safety-update/ozempicv-semaglutide-and-saxenda-liraglutide-vigilance-required-due-to-potentially-harmful-falsified-products.
- ↑ "European regulator warns EU, UK about fake Ozempic pens". Reuters. 18 October 2023. https://www.reuters.com/business/healthcare-pharmaceuticals/eu-medicines-regulator-warns-eu-uk-fake-ozempic-pens-2023-10-18/.
- ↑ "Several hospitalised in Austria after using suspected fake diabetes drug". The Guardian. Reuters. 24 October 2023. https://www.theguardian.com/world/2023/oct/24/several-hospitalised-in-austria-after-using-suspected-fake-diabetes-drug.
- ↑ "FDA warns consumers not to use counterfeit Ozempic (semaglutide) found in U.S. drug supply chain". 21 December 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-use-counterfeit-ozempic-semaglutide-found-us-drug-supply-chain.
- ↑ "Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss". U.S. Food and Drug Administration (FDA). 21 December 2023. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/medications-containing-semaglutide-marketed-type-2-diabetes-or-weight-loss.
- ↑ "Impact of semaglutide on biochemical and radiologic measures of metabolic-dysfunction associated fatty liver disease across the spectrum of glycaemia: A meta-analysis". Diabetes & Metabolic Syndrome 16 (6): 102539. June 2022. doi:10.1016/j.dsx.2022.102539. PMID 35709586.
- ↑ "Semaglutide and cancer: A systematic review and meta-analysis..". Diabetes Metab Syndr 17 (9): 102834. July 2023. doi:10.1016/j.dsx.2023.102834. PMID 37531876.
- ↑ "Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes". The New England Journal of Medicine 385 (6): 503–515. August 2021. doi:10.1056/NEJMoa2107519. PMID 34170647.
- ↑ "Ozempic, Drugs for Weight Loss Being Reviewed for Links to Suicide Risk". 27 July 2023. https://people.com/ozempic-wegovy-weight-loss-drug-suicide-risks-uk-7566473.
- ↑ "Weight-loss jabs investigated for suicide risk". BBC News. 10 July 2023. https://www.bbc.com/news/health-66119059.
- ↑ "FDA reports no link between weight-loss drugs and suicidal thoughts". The Washington Post. https://www.washingtonpost.com/health/2024/01/11/fda-ozempic-wegovy-suicide-safety/.
- ↑ "Update on FDA's ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity". FDA. 11 January 2024. https://www.fda.gov/drugs/drug-safety-and-availability/update-fdas-ongoing-evaluation-reports-suicidal-thoughts-or-actions-patients-taking-certain-type.
External links
 |



