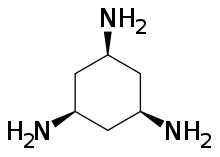
Chemistry:Cis,cis-1,3,5-Triaminocyclohexane
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1s,3s,5s)-Cyclohexane-1,3,5-triamine | |
| Other names
TACH; cis,cis-Cyclohexane-1,3,5-triamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H15N3 | |
| Molar mass | 129.207 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 285 °C (545 °F; 558 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
cis,cis-1,3,5-Triaminocyclohexane is an organic compound with the formula (CH2CHNH2)3. It is a triamine. Of the many isomers possible for triaminocyclohexane, the cis,cis-1,3,5-derivative has attracted attention because it is a common tripodal ligand, abbreviated as tach. It is a colorless oil. It is a popular tridentate ligand in coordination chemistry.
It is prepared from the triscarbamate of cyclohexane. The latter is generated via the Curtius rearrangement starting from cyclohexanetricarboxylic acid.[1]

Structure of [Ni(TACH)(H2O)3]2+ (color code: blue = nitrogen, red = oxygen; dark blue = nickel).[2]
Related ligands
- Tris(aminomethyl)ethane, another tripodal triamine (CH3C(CH2NH2]3)
References
- ↑ Bowen, Tom; Planalp, Roy P.; Brechbiel, Martin W. (1996). "An improved synthesis of cis,cis-1,3,5-triaminocyclohexane. Synthesis of novel hexadentate ligand derivatives for the preparation of gallium radiopharmaceuticals". Bioorganic & Medicinal Chemistry Letters 6 (7): 807–810. doi:10.1016/0960-894X(96)00110-2.
- ↑ Schwarzenbach, Gerold; Bürgi, Hans-Beat; Jensen, William P.; Lawrance, Geoffrey A.; Mønsted, Lene; Sargeson, Alan M. (1983). "Acid cleavage of nickel(II) complexes containing cis,cis-1,3,5-cyclohexanetriamine (TACH), crystal structure of [Ni(tach)(H2O)3](NO3)2, and a correlation between the structure and reactivity of nickel–polyamine complexes". Inorganic Chemistry 22 (26): 4029–4038. doi:10.1021/ic00168a042.
 |

