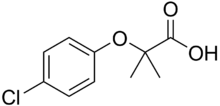
Chemistry:Clofibric acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(4-Chlorophenoxy)-2-methylpropanoic acid | |
| Other names
Clofibrin
Chlorofibrinic acid Chlorophenoxyisobutyric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H11ClO3 | |
| Molar mass | 214.645 g/mol |
| Appearance | White to yellow solid |
| Melting point | 118 to 123 °C (244 to 253 °F; 391 to 396 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Clofibric acid is a herbicide with the IUPAC name 2-(4-chlorophenoxy)-2-methylpropanoic acid and molecular formula C10H11ClO3. It functions as a plant growth regulator against the plant hormone auxin. Clofibric acid is also a naturally-occurring pharmaceutical compound,[1] having been found in Swiss lakes and the North Sea.[2]
Clofibric acid is a metabolite of the cholesterol-lowering pharmaceutical drug clofibrate, as well as that of the lipid regulators clofibrate, etofibrate, and theofibrate.[1]
Clofibric acid derivatives include etofibrate, pirifibrate, and picafibrate.
See also
References
- ↑ 1.0 1.1 Packer, Jennifer L; Werner, Jeffrey J; Latch, Douglas E; McNeill, Kristopher; Arnold, William A (2003). "Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen". Aquatic Sciences - Research Across Boundaries 65 (4): 342–351. doi:10.1007/s00027-003-0671-8.
- ↑ Buser, Hans-Rudolf; Müller, Markus D; Theobald, Norbert (1998). "Occurrence of the Pharmaceutical Drug Clofibric Acid and the Herbicide Mecoprop in Various Swiss Lakes and in the North Sea". Environmental Science & Technology 32: 188–192. doi:10.1021/es9705811.
 |

