Chemistry:Coibamide A
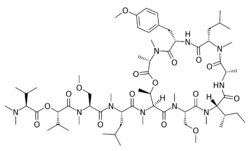
| |
| Names | |
|---|---|
| IUPAC name
[(2S)-1-[[(2S)-1-[[(2S)-1-[[(3S,6S,9S,12S,15S,18S,21S,22R)-15-[(2S)-butan-2-yl]-18-(methoxymethyl)-6-[(4-methoxyphenyl)methyl]-3,4,10,12,16,19,22-heptamethyl-9-(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1-oxa-4,7,10,13,16,19-hexazacyclodocos-21-yl]-methylamino]-4-methyl-1-oxopentan-2-yl]-methylamino]-3-methoxy-1-oxopropan-2-yl]-methylamino]-3-methyl-1-oxobutan-2-yl] (2S)-2-(dimethylamino)-3-methylbutanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C65H110N10O16 | |
| Molar mass | 1287.649 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Coibamide A is an antiproliferative depsipeptide which was isolated from a marine Leptolyngbya cyanobacterium.[1] Testing of coibamide A in the National Cancer Institute in vitro 60 human tumor cell line panel (NCI-60) revealed potent anti-proliferative activity and a unique selectivity profile. Similarities between coibamide A- and apratoxin A-induced changes in cell morphology, decreases in VEGFR2 expression and macroautophagy signaling in HUVECs raise the possibility that both cyanobacterial natural products share a common mechanism of action.[2] Wild-type mouse embryonic fibroblasts were more vulnerable to coibamide A than cells lacking autophagy-related protein 5 (Atg5) that suggest coibamide A as a compound with characteristics that may utilize autophagy for pro-death signaling.[3]
Solid-phase total syntheses of highly methylated cyclic azacoibamide A and its O-desmethyl analog were achieved to improve pharmacokinetic properties of coibamide A.[4]
References
- ↑ Medina, Rebecca A.; Goeger, Douglas E.; Hills, Patrice; Mooberry, Susan L.; Huang, Nelson; Romero, Luz I.; Ortega-Barría, Eduardo; Gerwick, William H. et al. (2008). "Coibamide A, a Potent Antiproliferative Cyclic Depsipeptide from the Panamanian Marine Cyanobacterium Leptolyngbyasp". Journal of the American Chemical Society 130 (20): 6324–5. doi:10.1021/ja801383f. PMID 18444611.
- ↑ Jeffrey D. Serrill; Xuemei Wan; Andrew M. Hau; Hyo Sang Jang; Daniel J. Coleman; Arup K. Indra; Adam W. G. Alani; Kerry L. McPhail et al. (2016). "Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts". Investigational New Drugs 34 (1): 24–40. doi:10.1007/s10637-015-0303-x. PMID 26563191.
- ↑ Tan, M. (2015). Investigation of autophagy-assisted cell death in response to the cancer cell toxin coibamide A (Doctoral dissertation).
- ↑ Sable, Ganesh A.; Park, Jaekwan; Lim, Soo-Jeong; Lim, Dongyeol (2016). "Solid-phase Total Synthesis of Amide Analogues of Coibamide A: Azacoibamide a andO-Desmethyl Azacoibamide A". Bulletin of the Korean Chemical Society 37 (3): 330–334. doi:10.1002/bkcs.10674.
 |

