Chemistry:Lyngbyatoxin-a
| |
| Names | |
|---|---|
| Systematic IUPAC name
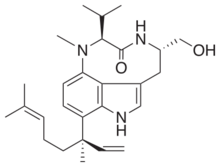
(2S,5S)-9-[(3R)-3,7-Dimethylocta-1,6-dien-3-yl]-5-(hydroxymethyl)-1-methyl-2-(propan-2-yl)-1,2,4,5,6,8-hexahydro-3H-[1,4]diazonino[7,6,5-cd]indol-3-one | |
| Other names
Lyngbyatoxin-a
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H39N3O2 | |
| Molar mass | 437.628 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, most notably Moorea producens (formerly Lyngbya majuscula). It is produced as defense mechanism to ward off any would-be predators of the bacterium, being a potent blister agent as well as carcinogen. Low concentrations cause a common skin condition known as seaweed dermatitis.[1][2][3][4][5][6]
Biosynthesis
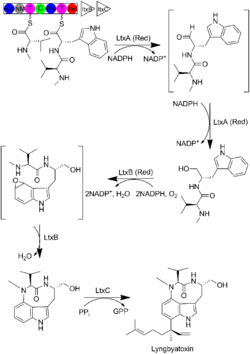
Lyngbyatoxin is a terpenoid indole alkaloid that belongs to the class of non-ribosomal peptides (NRP).[7] Lyngbyatoxin contains a nucleophilic indole ring that takes part in the activation of protein kinases. Figure 1, shows the biosynthesis of Lyngbyatoxin reported by Neilan et al. and Gerwick et al. The non-ribosomal peptide synthase (NRPS) LtxA protein condenses L-methyl-valine and L-tryptophan to form the linear dipeptide N-methyl-L-valyl-L-tryptophan. The latter is released via a NADPH-dependent reductive cleavage to form the aldehyde which is subsequently reduced to the corresponding alcohol. Then LtxB which is a P450-dependent monooxygenase serves as a catalyst in the oxidation and subsequent cyclization of N-methyl-L-valyl-L-tryptophan. Finally, LtxC which is a reverse prenyltransferase performs the transfer of a geranyl pyrophosphate (GPP) to carbon-7 of the indole ring which is accompanied by the loss of pyrophosphate.
References
- ↑ Cardellina, JH; Marner, FJ; Moore, RE (Apr 1979). "Seaweed dermatitis: structure of lyngbyatoxin A.". Science 204 (4389): 193–5. doi:10.1126/science.107586. PMID 107586.
- ↑ Fujiki, H; Mori, M; Nakayasu, M; Terada, M; Sugimura, T; Moore, RE (Jun 1981). "Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters". Proceedings of the National Academy of Sciences USA 78 (6): 3872–6. doi:10.1073/pnas.78.6.3872. PMID 6791164.
- ↑ Kozikowski, AP; Shum, PW; Basu, A; Lazo, JS (Aug 1991). "Synthesis of structural analogues of lyngbyatoxin A and their evaluation as activators of protein kinase C.". Journal of Medicinal Chemistry 34 (8): 2420–30. doi:10.1021/jm00112a017. PMID 1875340.
- ↑ Osborne, NJ; Webb, PM; Shaw, GR (Nov 2001). "The toxins of Lyngbya majuscula and their human and ecological health effects". Environment International 27 (5): 381–92. doi:10.1016/s0160-4120(01)00098-8. PMID 11757852.
- ↑ Ito, E; Satake, M; Yasumoto, T (May 2002). "Pathological effects of lyngbyatoxin A upon mice". Toxicon 40 (5): 551–6. doi:10.1016/s0041-0101(01)00251-3. PMID 11821127.
- ↑ Edwards, DJ; Gerwick, WH (2004). "Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase". Journal of the American Chemical Society 126 (37): 11432–3. doi:10.1021/ja047876g. PMID 15366877.
- ↑ Ongley, SE; Bian, X; Zhang, Y; Chau, R; Gerwick, WH; Müller, R; Neilan, BA (2013). "High-Titer Heterologous Production in E. coli of Lyngbyatoxin, a Protein Kinase C Activator from an Uncultured Marine Cyanobacterium". ACS Chemical Biology 8 (9): 1888–1893. doi:10.1021/cb400189j. PMID 23751865.
 |

