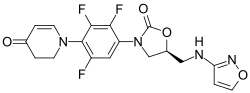
Chemistry:Contezolid
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Youxitai |
| Other names | MRX-I |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H15F3N4O4 |
| Molar mass | 408.337 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Contezolid (trade name Youxitai) is an antibiotic of the oxazolidinone class.[1][2] It is effective against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Streptococcus agalactiae, and other bacteria.[3]
In 2021, it was approved by the National Medical Products Administration of China for the treatment of complicated skin and soft tissue infections (cSSTI).[3][4]
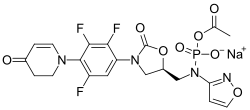
A prodrug of contezolid, contezolid acefosamil, which is formulated for IV administration[5] is in Phase III clinical trials for diabetic foot infection.[6]
References
- ↑ "New Potent Antibacterial Oxazolidinone (MRX-I) with an Improved Class Safety Profile". Journal of Medicinal Chemistry 57 (11): 4487–4497. June 2014. doi:10.1021/jm401931e. PMID 24694071.
- ↑ "A Phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections". The Journal of Antimicrobial Chemotherapy 77 (6): 1762–1769. May 2022. doi:10.1093/jac/dkac073. PMID 35265985.
- ↑ 3.0 3.1 "Contezolid: First Approval". Drugs 81 (13): 1587–1591. September 2021. doi:10.1007/s40265-021-01576-0. PMID 34365606.
- ↑ "Micurx wins China approval for antibacterial contezolid". BioWorld. 3 June 2021. https://www.bioworld.com/articles/507790-micurx-wins-china-approval-for-antibacterial-contezolid.
- ↑ "Discovery of Antibacterial Contezolid Acefosamil: Innovative O-Acyl Phosphoramidate Prodrug for IV and Oral Therapies". ACS Medicinal Chemistry Letters 13 (7): 1030–1035. July 2022. doi:10.1021/acsmedchemlett.2c00191. PMID 35859881.
- ↑ "Contezolid acefosamil by MicuRx Pharmaceuticals for Diabetic Foot Infection (DFI): Likelihood of Approval". GlobalData. 31 May 2023. https://www.pharmaceutical-technology.com/data-insights/contezolid-acefosamil-micurx-pharmaceuticals-diabetic-foot-infection-dfi-likelihood-of-approval.
 |