Chemistry:Copper naproxen
From HandWiki
 Sample of copper naproxen in a glass vial
| |
| |
| Names | |
|---|---|
| Other names
Copper(II) 6-methoxy-a-methyl-2-naphthaleneacetate
| |
| Identifiers | |
| Properties | |
| C28H26CuO6 | |
| Molar mass | 522.056 g·mol−1 |
| Appearance | Green solid |
| Solubility | Soluble in methanol, 1,4-dioxane,[1] DMSO, DMF[2] |
| Related compounds | |
Related compounds
|
Copper aspirinate Copper ibuprofenate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
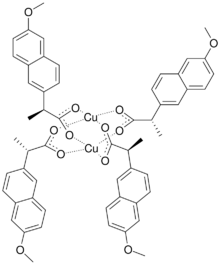
Copper naproxen is a chemical complex of copper2+ chelated with the anti-inflammatory drug naproxen.[1] Copper complexes of NSAIDs like naproxen have been shown to have greater anti-inflammatory properties than the base drug.[3][2]
Copper naproxen can by found as a monohydrate, and it can form complexes with other organic molecules such as nicotinyl alcohol, 3-methylpyridine, and caffeine.[1][4][5]
Preparation
Copper naproxen is prepared by reacting sodium naproxen with a copper(II) salt such as copper(II) sulfate.[1]
[math]\ce{ 2C14H13NaO3 + CuSO4 -> C28H26CuO6 + Na2SO4 }[/math]
References
- ↑ 1.0 1.1 1.2 1.3 Melnı́k, Milan; Macásková, Lubov; Holloway, Clive E; Mrozinski, Jerzy; Kalin’ska, Bozena (March 2000). "Spectral and magnetic properties of copper(II) naproxenates" (in en). Inorganica Chimica Acta 299 (2): 284–287. doi:10.1016/S0020-1693(99)00504-6. https://linkinghub.elsevier.com/retrieve/pii/S0020169399005046.
- ↑ 2.0 2.1 Dimiza, Filitsa; Perdih, Franc; Tangoulis, Vassilis; Turel, Iztok; Kessissoglou, Dimitris P.; Psomas, George (March 2011). "Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: Synthesis, structure, DNA- and albumin-binding" (in en). Journal of Inorganic Biochemistry 105 (3): 476–489. doi:10.1016/j.jinorgbio.2010.08.013. https://linkinghub.elsevier.com/retrieve/pii/S0162013410002060.
- ↑ Sorenson, John R.J. (1989) (in en), 6 Copper Complexes Offer a Physiological Approach to Treatment of Chronic Diseases, Progress in Medicinal Chemistry, 26, Elsevier, pp. 437–568, doi:10.1016/s0079-6468(08)70246-7, ISBN 978-0-444-81038-0, https://linkinghub.elsevier.com/retrieve/pii/S0079646808702467, retrieved 2022-04-10
- ↑ Caglar, Sema; Adiguzel, Ekrem; Sariboga, Bahtiyar; Temel, Ersin; Buyukgungor, Orhan (2014-02-16). "Mono and dinuclear copper(II) naproxenato complexes containing 3-picoline and 4-picoline: synthesis, structure, properties, catechol oxidase, and antimicrobial activities" (in en). Journal of Coordination Chemistry 67 (4): 670–683. doi:10.1080/00958972.2014.891198. ISSN 0095-8972. https://www.tandfonline.com/doi/full/10.1080/00958972.2014.891198.
- ↑ Abuhijleh, A. Latif; Khalaf, Juhienah (September 2010). "Copper (II) complexes of the anti-inflammatory drug naproxen and 3-pyridylmethanol as auxiliary ligand. Characterization, superoxide dismutase and catecholase – mimetic activities" (in en). European Journal of Medicinal Chemistry 45 (9): 3811–3817. doi:10.1016/j.ejmech.2010.05.031. PMID 20605277. https://linkinghub.elsevier.com/retrieve/pii/S0223523410003715.
 |

