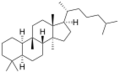
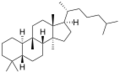
Chemistry:Cucurbitane
Cucurbitane is a tetracyclic chemical compound with formula C30H54. It is a polycyclic hydrocarbon, specifically triterpene. It is also an isomer of lanostane (specifically 19(10→9β)-abeolanostane), from which it differs by the formal shift of a methyl group (carbon number 19) from the 10 to the 9β position in the standard steroid numbering scheme.[1][2]
The name is applied to two stereoisomers, distinguished by the prefixes 5α- and 5β-, which differ by the handedness of the bonds at a particular carbon atom (number 5 in the standard steroid numbering scheme).[1]
-
5α-Cucurbitane
-
5β-Cucurbitane
Cucurbitane is the core chemical structure of a class of derivatives known as cucurbitane-type triterpenoids or simply as cucurbitanes.[3]
Derivatives
Natural compounds
Compounds with the basic cucurbitane skeleton are found in many plants, and some are important phytopharmaceuticals.[4] Natural cucurbitane-related compounds include:
Named
- Balsaminapentaol, from Momordica balsamina.[5]
- Balsaminol A, from Momordica balsamina.[5]
- Balsaminol B, from Momordica balsamina.[5]
- Brydioside A from Bryonia dioica[4]: 64
- Bryoamaride and derivatives from Bryonia dioica[4]: 65, 66
- Charantin or foetidin, from Momordica charantia[6] and Momordica foetida[7]
- Charantosides I-VIII, from Momordica charantia.[8]
- Cucurbalsaminol B, from Momordica balsamina.[5]
- Cucurbalsaminol A, from Momordica balsamina.[5]
- Cucurbitacins A-L, O-T[4][9][10]: 3–8
- Datiscosides, from Datisca glomerata[4]: 16–19
- Endecaphyllacins A and B, from roots of Hemsleya endecaphylla[10]: 1, 2
- Hemslecins A and B, from roots of H. endecaphylla[10]
- Lepidolide, from the mushroom Russula lepida[11]
- Karavilagenin E, from Momordica balsamina.[5]
- Khekadaengosides A, B, D and K, from Trichosanthes tricuspidata[4]: 57, 58, 67, 68
- Kuguacins A-S, from stems and leaves of Momordica charantia[12][13]
- Kuguaglycosides A-H, from the root of Momordica charantia[14]
- Mogrosides I-V, from the fruits of Siraitia grosvenorii[15]
- Momordicin I, II and 28, from Momordica charantia[16][17]
- Momordicines II and IV, from leaves of Momordica charantia[18]
- Momordicosides A-S, from Momordica charantia fruits[8][19][20]
- Neokuguaglucoside, from Momordica charantia fruits[21]
- Neomogroside, from the fruit of Siraitia grosvenorii.[22]
- Pentanorcucurbitacins A and B[23]: 1, 2
- Perseapicroside A, from Persea mexicana[4]: 44
- Scandenoside R9, from Hemsleya panacis-scandens[4]: 45
- Spinosides A and B, from Desfontainia spinosa[4]: 61, 62
Unnamed
- 3β,7β,23ξ-trihydroxycucurbita-5,24-dien-19-al, soluble in chloroform, melts at 123−125 °C, from Momordica charantia, Momordica foetida.[24]: 1
- 3β,7β,25-trihydroxycucurbita-5,23-dien-19-al, soluble in chloroform, melts at 188−191 °C, from Momordica charantia, Momordica foetida[24]: 2
- 3β,7β-dihydroxy-25-methoxycucurbita-5,23-dien-19-al, soluble in chloroform, from Momordica charantia, Momordica foetida[24]: 3
- 5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β,19-diol, soluble in chloroform, melts at 182−184 °C, from Momordica foetida[24]: 4
- 5β,19-epoxycucurbita-6,23-dien-3β,19,25-triol, soluble in chloroform, from Momordica foetida[24]: 5
- 5β,19-epoxy-19-methoxycucurbita-6,23-dien-3β,25-diol, soluble in chloroform, melts at 102−104 °C, from Momordica charantia, Momordica foetida[24]: 6
- 5β,19-epoxy-19,25-dimethoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, from Momordica charantia, Momordica foetida[24]: 7
- 5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, melts at 139−141 °C, from Momordica charantia, Momordica foetida[24]: 8
- , C40H66O9, white powder soluble in methanol, from Momordica charantia fruit (8 mg/35 kg)[19]: 1
- 23-O-β-allopyranosylecucurbita-5,24-dien-7α,3β,22(R),23(S)-tetraol 3-O-β-allopyranoside,C42H69O14, white powder soluble in methanol, from Momordica charantia fruit (10 mg/35 kg)[19]: 2
- 23(R),24(S),25-trihydroxycucurbit-5-ene 3-O-{[β-glucopyranosyl(1→6)]-O-β-glucopyranosyl}-25-O-β-glucopyranoside, C48H82O19, white powder soluble in methanol, from Momordica charantia fruit (10 mg/35 kg)[19]: 3
- 2,16-dihydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 2-O-β-D-glucopyranoside, soluble in ethanol, from Cucurbita pepo fruits (25 mg/15 kg)[9]: 3
- 16-hydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, white powder, soluble in ethanol, from Cucurbita pepo fruits (12 mg/15 kg)[9]: 4
- 7-methoxycucurbita-5,24-diene-3β,23(R)-diol, from Momordica balsamina[25]
- 25,26,27-trinorcucurbit-5-ene-3,7,23-trione C27H40O3, white powder, soluble in methanol, from stems of Momordica charantia (6 mg/18 kg)[23]: 3
See also
- Goyaglicoside
- Karaviloside
- Momordenol, from Momordica charantia[16]
- 24(R)-stigmastan-3β,5α,6β-triol-25-ene 3-O-β-glucopyranoside, C35H60O8, white powder, from Momordica charantia fruit (15 mg/35 kg)[19]: 4
References
- ↑ 1.0 1.1 "The Nomenclature of Steroids — Revised Tentative Rules". European Journal of Biochemistry 10: 1–19. 1969. doi:10.1111/j.1432-1033.1969.tb00650.x.
- ↑ Satish Kumar and Raj Kumar (1991), Dictionary of Biochemistry. Anmol Publications, India
- ↑ Jun Ma, Paul Whittaker, Amy C. Keller, Eugene P. Mazzola, Rahul S. Pawar, Kevin D. White, John H. Callahan, Edward J. Kennelly, Alexander J. Krynitsky, Jeanne I. Rader (2010). "Cucurbitane-Type Triterpenoids from Momordica charantia". Planta Med 76 (15): 1758-1761. doi:10.1055/s-0030-1249807.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Chen, J. C.; Chiu, M. H.; Nie, R. L.; Cordell, G. A.; Qiu, S. X. (2005). "Cucurbitacins and cucurbitane glycosides: Structures and biological activities". Natural Product Reports 22 (3): 386–399. doi:10.1039/B418841C. PMID 16010347.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Ramalhete, C. T.; Mansoor, T. A.; Mulhovo, S.; Molnár, J.; Ferreira, M. J. U. (2009). "Cucurbitane-Type Triterpenoids from the African PlantMomordica balsamina". Journal of Natural Products 72 (11): 2009–2013. doi:10.1021/np900457u. PMID 19795842.
- ↑ Lolitkar, M. M.; Rao, M. R. Rajarama (1962). "Note on a Hypoglycaemic Principle Isolated from the fruits of Momordica charantia". Journal of the University of Bombay 29: 223–224.
- ↑ Olaniyi, A. A. (1975). "A neutral constituent of Momordica foetida". Lloydia 38 (4): 361–362. PMID 1186439.
- ↑ 8.0 8.1 Akihisa, T.; Higo, N.; Tokuda, H.; Ukiya, M.; Akazawa, H.; Tochigi, Y.; Kimura, Y.; Suzuki, T. et al. (2007). "Cucurbitane-Type Triterpenoids from the Fruits of Momordica charantia and Their Cancer Chemopreventive Effects". Journal of Natural Products 70 (8): 1233–1239. doi:10.1021/np068075p. PMID 17685651.
- ↑ 9.0 9.1 9.2 Wang, Da-Cheng; Pan, Hong-Yu; Deng, Xu-Ming; Xiang, Hua; Gao, Hui-Yuan; Cai, Hui; Wu, Li-Jun (2007). "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua". Journal of Asian Natural Products Research 9 (6): 525–529. doi:10.1080/10286020600782538. PMID 17885839.
- ↑ 10.0 10.1 10.2 Chen, J. C.; Zhang, G. H.; Zhang, Z. Q.; Qiu, M. H.; Zheng, Y. T.; Yang, L. M.; Yu, K. B. (2008). "Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers ofHemsleya endecaphyllawith HIV-1 Inhibitory Activity". Journal of Natural Products 71 (1): 153–155. doi:10.1021/np0704396. PMID 18088099.
- ↑ Jian-Wen Tan, Ze-Jun Dong, Zhi-Hui Ding and Ji-Kai Liu (2002), "Lepidolide, a Novel Seco-ring-A Cucurbitane Triterpenoid from Russula lepida (Basidiomycetes)". Zeitschrift für Naturforschung Series C, volume 57C issue 11/12, pages 963-965.
- ↑ Chen, J. C.; Liu, W. Q.; Lu, L.; Qiu, M. H.; Zheng, Y. T.; Yang, L. M.; Zhang, X. M.; Zhou, L. et al. (2009). "Kuguacins F–S, cucurbitane triterpenoids from Momordica charantia". Phytochemistry 70 (1): 133–140. doi:10.1016/j.phytochem.2008.10.011. PMID 19041990.
- ↑ Chen, J. C.; Tian, R. R.; Qiu, M. H.; Lu, L.; Zheng, Y. T.; Zhang, Z. Q. (2008). "Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia". Phytochemistry 69 (4): 1043–1048. doi:10.1016/j.phytochem.2007.10.020. PMID 18045630.
- ↑ Chen, J. C.; Lu, L.; Zhang, X. M.; Zhou, L.; Li, Z. R.; Qiu, M. H. (2008). "Eight New Cucurbitane Glycosides, Kuguaglycosides A – H, from the Root ofMomordica charantia L". Helvetica Chimica Acta 91 (5): 920. doi:10.1002/hlca.200890097.
- ↑ Takasaki, Midori; Konoshima, Takao; Murata, Yuji; Sugiura, Masaki; Nishino, Hoyoku; Tokuda, Harukuni; Matsumoto, Kazuhiro; Kasai, Ryoji et al. (2003). "Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori". Cancer Letters 198 (1): 37–42. doi:10.1016/s0304-3835(03)00285-4. PMID 12893428.
- ↑ 16.0 16.1 Begum, Sabira; Ahmed, Mansour; Siddiqui, Bina S.; Khan, Abdullah; Saify, Zafar S.; Arif, Mohammed (1997). "Triterpenes, A Sterol and a Monocyclic Alcohol From Momordica Charantia". Phytochemistry 44 (7): 1313–1320. doi:10.1016/s0031-9422(96)00615-2.
- ↑ Fatope, Majekodunmi; Takeda, Yoshio; Yamashita, Hiroyasu; Okabe, Hikaru; Yamauchi, Tatsuo (1990). "New cucurbitane trirterpenoids from Momordica charantia". Journal of Natural Products 53 (6): 1491–1497. doi:10.1021/np50072a014.
- ↑ Daniel Bisrat Mekuria, Takehiro Kashiwagi, Shin-ichi Tebayashi, and Chul-Sa Kim (2006)"Cucurbitane Glucosides from Momordica charantia Leaves as Oviposition Deterrents to the Leafminer, Liriomyza trifolii". Z. Naturforsch., volume 61c, pages 81–86
- ↑ 19.0 19.1 19.2 19.3 19.4 Liu, J. Q.; Chen, J. C.; Wang, C. F.; Qiu, M. H. (2009). "New Cucurbitane Triterpenoids and Steroidal Glycoside from Momordica charantia". Molecules 14 (12): 4804–4813. doi:10.3390/molecules14124804. PMID 20032860.
- ↑ Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. (2006). "Momordica charantia Constituents and Antidiabetic Screening of the Isolated Major Compounds". Chemical & Pharmaceutical Bulletin 54 (7): 1017–1021. doi:10.1248/cpb.54.1017. PMID 16819222.
- ↑ Liu, J. Q.; Chen, J. C.; Wang, C. F.; Qiu, M. H. (2010). "One new cucurbitane triterpenoid from the fruits of Momordica charantia". European Journal of Chemistry 1 (4): 294. doi:10.5155/eurjchem.1.4.294-296.131.
- ↑ Si Jian-yong, Chen Di-hua, Chang Qi and Shen Lian-gang (1996), Isolation and Determination of Cucurbitane-Glycosides from Fresh Fruits of Siraitia Grosvenorii. Journal of Integrative Plant Biology, volume 38, issue 6, pages, 489–494
- ↑ 23.0 23.1 Chen, C. R.; Liao, Y. W.; Wang, L.; Kuo, Y. H.; Liu, H. J.; Shih, W. L.; Cheng, H. L.; Chang, C. I. (2010). "Cucurbitane Triterpenoids from Momordica charantia and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells". Chemical & Pharmaceutical Bulletin 58 (12): 1639–1642. doi:10.1248/cpb.58.1639. PMID 21139270.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 Mulholland, D. A.; Sewram, V.; Osborne, R.; Pegel, K. H.; Connolly, J. D. (1997). "Cucurbitane triterpenoids from the leaves of Momordica foetida". Phytochemistry 45 (2): 391. doi:10.1016/S0031-9422(96)00814-X.
- ↑ Spengler, Gabriella; Ramalhete, Cátia; Martins, Marta; Martins, Ana; Serly, Julianna; Viveiros, Miguel; Molnár, Joseph; Duarte, Noélia et al. (2010). "Evaluation of Cucurbitane-type Triterpenoids from Momordica balsamina on P-Glycoprotein (ABCB1) by Flow Cytometry and Real-time Fluorometry". Anticancer Research 30: 4867–4871.
Template:Terpenes Template:Tetracyclics
 |