Chemistry:Cumyl alcohol
| |
| Names | |
|---|---|
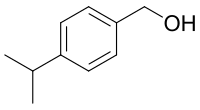
| IUPAC name
(4-Propan-2-ylphenyl)methanol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.221 g·mol−1 |
| Odor | Caraway |
| Density | 0.974-0.982 |
| Melting point | 28 °C (82 °F; 301 K) |
| log P | 2.370 |
Refractive index (nD)
|
1.518-1.525 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+317Script error: No such module "Preview warning".Category:GHS errors, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cumyl alcohol, also called 4-isopropylbenzyl alcohol, is a liquid, hydroxy functional, aromatic organic chemical with formula C10H14O. It has the CAS Registry Number of 536-60-7 and the IUPAC name of (4-propan-2-ylphenyl)methanol.[2][3] It is REACH registered with the EC number of 208-640-4.[4]
Uses
The most common use is as a food additive to add flavor.[5][6] The material also has insect repellent properties.[7]
Manufacture
Hydrogenation of cuminal can produce cumyl alcohol.[8]
Other
Cumyl alcohol is an undesired side reaction product when LDPE is crosslinked.[9] LDPE is used as a plastic electric insulator for electrical power cables.[10] The cumyl alcohol reduces the insulating properties.[11][12]
Alternative names
Main sources of information.[13][14]
- p-Cymen-7-ol
- 4-isopropylbenzyl alcohol
- Cumic alcohol[15]
- Cuminol
- Cuminyl alcohol[16]
- (4-Isopropylphenyl)methanol
- Cuminic alcohol
Toxicology
The toxicity of the material has been studied and is reasonably well understood.[17][18][19]
References
- ↑ "4-Isopropylbenzyl alcohol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/325#section=Safety-and-Hazards.
- ↑ PubChem. "4-Isopropylbenzyl alcohol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/325.
- ↑ "4-isopropylbenzyl alcohol" (in en). https://www.wikidata.org/wiki/Q1143803.
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.007.857.
- ↑ "P-ISOPROPYLBENZYL ALCOHOL | FEMA". https://www.femaflavor.org/flavor-library/p-isopropylbenzyl-alcohol.
- ↑ "Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Flavouring Group Evaluation 20 (FGE.20): Benzyl alcohols, benzaldehydes, a related acetal, benzoic acids, and related esters from chemical group 23 | EFSA" (in en). 9 January 2006. https://www.efsa.europa.eu/en/efsajournal/pub/296.
- ↑ "4-Isopropylbenzyl alcohol | CAS 536-60-7 | SCBT - Santa Cruz Biotechnology". https://www.scbt.com/p/4-isopropylbenzyl-alcohol-536-60-7.
- ↑ Cooke, R. G.; Gillespie, D. T.; Macbeth, A. Killen (1938-01-01). "338. Cumyl alcohol" (in en). Journal of the Chemical Society (Resumed): 1825–1826. doi:10.1039/JR9380001825. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1938/jr/jr9380001825.
- ↑ Hirai, N.; Maeno, Y.; Tanaka, T.; Ohki, Y.; Okashita, M.; Maeno, T. (October 2003). "Roles of cumyl alcohol and crosslinked structure in homo-charge trapping in crosslinked polyethylene". 2003 Annual Report Conference on Electrical Insulation and Dielectric Phenomena. pp. 213–216. doi:10.1109/CEIDP.2003.1254831. ISBN 0-7803-7910-1. https://ieeexplore.ieee.org/document/1254831.
- ↑ Gulmine, J. V.; Akcelrud, L. (2006-10-01). "FTIR characterization of aged XLPE" (in en). Polymer Testing 25 (7): 932–942. doi:10.1016/j.polymertesting.2006.05.014. ISSN 0142-9418. https://www.sciencedirect.com/science/article/pii/S0142941806001061.
- ↑ Chen, Meng; Zhang, Hongliang; Wang, Yalin; Wu, Jiandong; Yin, Yi (February 2020). "Space charge dynamics of acetophenone and cumyl alcohol and their synergistic effect in LDPE". IEEE Transactions on Dielectrics and Electrical Insulation 27 (1): 67–75. doi:10.1109/TDEI.2019.008320. ISSN 1070-9878. https://ieeexplore.ieee.org/document/8985656.
- ↑ Hussin, N.; Chen, G. (February 2012). "Analysis of space charge formation in LDPE in the presence of crosslinking byproducts". IEEE Transactions on Dielectrics and Electrical Insulation 19 (1): 126–133. doi:10.1109/TDEI.2012.6148510. ISSN 1070-9878. https://ieeexplore.ieee.org/document/6148510.
- ↑ "cuminyl alcohol, 536-60-7" (in en-US). http://www.thegoodscentscompany.com/data/rw1016451.html.
- ↑ "p-Cymen-7-ol" (in en). https://webbook.nist.gov/cgi/cbook.cgi?InChI=1/C10H14O/c1-8(2)10-5-3-9(7-11)4-6-10/h3-6,8,11H,7H2,1-2H3.
- ↑ "Cumic alcohol | CAS#536-60-7 | insulinotropic agent | MedKoo". https://medkoo.com/products/23231.
- ↑ "cuminyl alcohol, 536-60-7" (in en-US). http://www.thegoodscentscompany.com/data/rw1016451.html.
- ↑ Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A. et al. (July 2020). "RIFM fragrance ingredient safety assessment, p-isopropylbenzyl alcohol, CAS Registry Number 536-60-7" (in en). Food and Chemical Toxicology 141: 111338. doi:10.1016/j.fct.2020.111338. PMID 32335211. https://linkinghub.elsevier.com/retrieve/pii/S027869152030226X.
- ↑ Patil, Swapnil B.; Takalikar, Shreehari S.; Joglekar, Madhav M.; Haldavnekar, Vivek S.; Arvindekar, Akalpita U. (October 2013). "Insulinotropic and β-cell protective action of cuminaldehyde, cuminol and an inhibitor isolated from Cuminum cyminum in streptozotocin-induced diabetic rats" (in en). British Journal of Nutrition 110 (8): 1434–1443. doi:10.1017/S0007114513000627. ISSN 0007-1145. PMID 23507295. https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/insulinotropic-and-cell-protective-action-of-cuminaldehyde-cuminol-and-an-inhibitor-isolated-from-cuminum-cyminum-in-streptozotocininduced-diabetic-rats/557CFBF0890CFE2C25DC051914635170.
- ↑ Ali, Mohd Sajid; Rehman, Md Tabish; Al-Lohedan, Hamad A.; AlAjmi, Mohamed Fahad (2022-12-12). "Exploration of the binding between cuminol and bovine serum albumin through spectroscopic, molecular docking and molecular dynamics methods". Journal of Biomolecular Structure and Dynamics 40 (22): 12404–12412. doi:10.1080/07391102.2021.1971560. ISSN 0739-1102. PMID 34488560. https://doi.org/10.1080/07391102.2021.1971560.
 |

