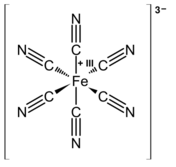
Chemistry:Cyanometalate
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands.[1] Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. The resulting cyanometallate anions are often used as building blocks for more complex structures called coordination polymers, the best known example of which is Prussian blue, a common dyestuff.[2]
Examples
Homoleptic cyanometallates
Homoleptic cyanometallates are complexes where the only ligand is cyanide. For transition metals, well known homoleptic cyanometallates are the hexacyanides. Hexacyanometalates are known for Ti(III), V(III), Cr(III), Cr(II), Mn(IV), Mn(III), Mn(II), Fe(II), Fe(III), Co(III), Ru(III), Ru(II), Os(III), and Os(II). Other more labile derivatives are also known. The Cr(II),[3] Mn(III), Mn(II), Fe(II), Fe(III), and Co(III) derivatives are low-spin, reflecting the strong binding of cyanide, i.e. cyanide ranks highly in the spectrochemical series when significant backbonding can occur. Since cyanide-anion has the largest σ-donation ability at its C-end, most soluble(molecular) metal-cyanide complexes have metal-carbon, rather than metal-ntrogen bonds.[4] With low d-electron counts, however, inversion of cyanometallates to nitrile complexes can occur. Lower metal oxidation states can be achieved with binding of Lewis acids to the terminal nitrogen lone pairs.
Pentacyanocobaltate ([Co(CN)
5]3−) is produced by the addition of five or more equivalents of a cyanide to a solution of a cobalt(II) salt. It is square pyramidal.[5] Solutions of [Co(CN)
5]−
3 undergo a variety of reactions, such as hydrogenation:[6]
- 2[Co(CN)
5]3− + H
2 → 2 [Co(CN)
5H]3−
Several tetracyanometalates are also known, the best known being those of the d8 metals, Ni(II), Pd(II), and Pt(II). These species are square-planar and diamagnetic. In addition to [Ni(CN)4]4−, nickel also forms [Ni2(CN)6]4-, with a Ni(I)-Ni(I) bond. The coinage metals form stable dicyanometallates, [Cu(CN)2]−, [Ag(CN)2]−, and [Au(CN)2]−. For heavier metals, other stoichiometries are known such as K4Mo(CN)8 and Potassium heptacyanorhenate. Some cyanometallates are clusters featuring metal-metal bonds, such as [Mo2(CN)8]4−.
| name | formula | formula weight | charge | oxidation
state |
comment | reference |
|---|---|---|---|---|---|---|
| Tetracyanidoborate | [B(CN)4]− | −1 | +3 | [7] | ||
| Si(CN)62– | −2 | +4 | [8] | |||
| [Ti(CN)4]2− | −2 | +2 | [9] | |||
| hexacyanotitanate | [Ti(CN)6]3− | −3 | +3 | orange | [9] | |
| [Ti(CN)7]4− | −4 | +3 | [9] | |||
| [Ti(CN)8]5− | −5 | +3 | dark green | [9][10] | ||
| [V(CN)6]4− | −4 | +2 | yellow brown | [10] | ||
| [V(CN)7]4− | −4 | +3 | scarlet purple | [10] | ||
| [Cr(CN)6]6− | −6 | 0 | dark green | [10] | ||
| [Cr(CN)6]3− | −3 | +3 | pale yellow | [10] | ||
| hexacyanomanganate | [MnIII(CN)6]3– | −3 | +3 | |||
| Hexacyanoferrate(II) | [FeII(CN)6]4− | −4 | +3 | [11] | ||
| Tricyanidoferrate(−IV) | [Fe(CN)3]7− | −7 | −4 | [12] | ||
| [Co(CN)3]6− | −6 | −3 | [12] | |||
| [Co(CN)6]3− | −3 | +3 | ||||
| Tetracyanonickelate(II) | [Ni(CN)4]2– | −2 | +2 | yellow orange | ||
| Tetracyanonickelate(II|) | [Ni(CN)4]– | −1 | +3 | |||
| hexacyanodickelate(I) | [Ni2(CN)6]4− | −4 | +1 | |||
| Ge(CN)62– | −2 | +4 | [8] | |||
| [Mo(CN)7]4− | −4 | +3 | dark green | [10] | ||
| [Mo(CN)8]4− | −4 | +4 | yellow | [13][10] | ||
| Tricyanidoruthenate(−IV) | [Ru(CN)3]7− | −7 | −4 | [12] | ||
| [Pd(CN)4]2– | −2 | +2 | [14] | |||
| dicyanidoargentate(I) | [Ag(CN)2]– | −1 | +1 | |||
| Sn(CN)62– | −2 | +4 | [8] | |||
| pentacyanido antimonate | [Sb(CN)5]2– | −2 | +3 | [15] | ||
| Heptacyanotungstate(IV) | [W(CN)7]3− | −3 | +4 | [16] | ||
| [W(CN)8]3− | −3 | +5 | [17] | |||
| [Re(CN)7]3– | −3 | +4 | [17] | |||
| [Pt(CN)4]2− | −2 | +2 | [18] | |||
| [Pt(CN)6]2– | −2 | +4 | [17] | |||
| dicyanidoaurate(I) | [Au(CN)2]– | −1 | +1 | |||
| tetracyanidoaurate(III) | [Au(CN)4]– | −1 | +3 | [19] | ||
| pentacyanido bismuthate | [Bi(CN)5]2– | −2 | +3 | [15] | ||
| hexacyanido bismuthate | [Bi(CN)6]3– | −3 | +3 | [15] | ||
| hendecacyanido dibismuthate | [Bi2(CN)11]5– | −5 | +3 | [15] |
Heteroleptic cyanometallates
Mixed ligand cyanometallates with anywhere from one to five cyanide ligands have been prepared. One example is the zero-valent [Fe(CO)4(CN)]−. Heteroleptic cyanometallates are of interest outside of the research laboratory, with one example being the drug sodium nitroprusside (Na2FeNO(CN)5). Other studies have demonstrated their competency as photoredox catalysts.
Synthesis
Because cyanide is a powerful nucleophile and a strong ligand, cyanometallates are generally prepared by the direct reaction of cyanide salts with simple metal salts. If other ligands are present on the metal, these are often displaced by cyanide. By far the largest application of cyanometalates is the production of [Au(CN)2]− in the extraction of gold from low grade ores. This conversion involves oxidation of metallic gold into Au+:
- 4 Au + 8 CN− + O2 + 2 H2O → 4 [Au(CN)2]− + 4 OH−
Reactions
Redox
Because the M-CN bond is strong and delocalizes electron density to the ligands, several cyanometallates exhibit multiple redox states. A well known couple is [Fe(CN)6]3−/4−. Mn(IV), Mn(III), and Mn(II) are known for hexacyanomanganate. Few unidentate ligands allow similar redox transformations wherein both members of the redox couple are observable in solution. Another perhaps more dramatic example is the 2 e– reduction of the square planar tetracyanonickelate to its tetrahedral Ni(0) derivative:
- [Ni(CN)4]2− + 2 e– → [Ni(CN)4]4−
N-Centered reactions
Many characteristic reactions of metal cyanides arise from ambidentate nature of cyanide, i.e. both the nitrogen and the carbon extremities of the anion are basic. Thus cyanometalates can be alkylated to give isocyanide complexes.[20] Cyanide ligands are susceptible to protonation, hence many cyanometalates are highly solvatochromic. The nitrogen terminus is a good ligand for other metals. The latter tendency is illustrated by the condensation of ferrocyanide salts with other metal ions to give polymers, such as Prussian blue. Such polymers feature Fe-CN-M linkages.
See also
- Transition metal nitrile complexes – coordination compounds containing nitrile ligands (coordinating via N)
References
- ↑ Sharpe, A. G. The Chemistry of Cyano Complexes of the Transition Metals; Academic Press: London, 1976. ISBN:0-12-638450-9.
- ↑ *Dunbar, K. R. and Heintz, R. A., "Chemistry of Transition Metal Cyanide Compounds: Modern Perspectives", Progress in Inorganic Chemistry, 1997, 45, 283-391.
- ↑ Eaton, Janice P.; Nicholls, David (1981). "The Complex Cyanides of Chromium(II) and Chromium(0)". Transition Metal Chemistry 6 (4): 203–206. doi:10.1007/BF00618223.
- ↑ Recent progress in transition metal hexacyanometallates: From structure to properties and functionality. 2022. Coordination Chemistry Reviews. 453/. Y. Avila, P. Acevedo-Peña, L. Reguera, E. Reguera. doi: 10.1016/j.ccr.2021.214274
- ↑ Brown, Leo D.; Raymond, Kenneth N. (1975). "Structural Characterization of the Pentacyanocobaltate(II) Anion in the Salt Tris(diethyldiisopropylammonium) Pentacyanocobaltate(II)". Inorganic Chemistry 14 (11): 2590–2594. doi:10.1021/ic50153a002.
- ↑ Kwiatek, Jack (1968). "Reactions Catalyzed by Pentacyanocobaltate(II)". Catalysis Reviews 1: 37–72. doi:10.1080/01614946808064700.
- ↑ Nitschke, Christian; Köckerling, Martin (March 2009). "A New Transition Metal Tetracyanidoborate: Synthesis, Structure and Properties of Co[B(CN) 4 2 ·2H 2 O"] (in en). Zeitschrift für anorganische und allgemeine Chemie 635 (3): 503–507. doi:10.1002/zaac.200801234. https://onlinelibrary.wiley.com/doi/10.1002/zaac.200801234.
- ↑ 8.0 8.1 8.2 Smallwood, Zoe M.; Davis, Martin F.; Hill, J. Grant; James, Lara J. R.; Portius, Peter (April 2019). "Syntheses, Structures, and Infrared Spectra of the Hexa(cyanido) Complexes of Silicon, Germanium, and Tin" (in en). Inorganic Chemistry 58 (7): 4583–4591. doi:10.1021/acs.inorgchem.9b00150. ISSN 0020-1669. https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b00150.
- ↑ 9.0 9.1 9.2 9.3 Nicholls, David; Ryan, T.Anthony (January 1980). "Complex cyanides of titanium" (in en). Inorganica Chimica Acta 41: 233–237. doi:10.1016/S0020-1693(00)88461-3. https://linkinghub.elsevier.com/retrieve/pii/S0020169300884613.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 Chadwick, B.M.; Sharpe, A.G. (1966), "Transition Metal Cyanides and Their Complexes" (in en), Advances in Inorganic Chemistry and Radiochemistry (Elsevier) 8: pp. 83–176, doi:10.1016/s0065-2792(08)60201-0, ISBN 978-0-12-023608-4, https://linkinghub.elsevier.com/retrieve/pii/S0065279208602010, retrieved 2024-01-21
- ↑ Buser, H. J.; Schwarzenbach, D.; Petter, W.; Ludi, A. (1977-11-01). "The crystal structure of Prussian Blue: Fe4[Fe(CN)63.xH2O"] (in en). Inorganic Chemistry 16 (11): 2704–2710. doi:10.1021/ic50177a008. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50177a008.
- ↑ 12.0 12.1 12.2 Jach, Franziska; Wagner, Frank R.; Amber, Zeeshan H.; Rüsing, Michael; Hunger, Jens; Prots, Yurii; Kaiser, Martin; Bobnar, Matej et al. (2021-07-12). "Tricyanidoferrates(−IV) and Ruthenates(−IV) with Non‐Innocent Cyanido Ligands" (in en). Angewandte Chemie International Edition 60 (29): 15879–15885. doi:10.1002/anie.202103268. ISSN 1433-7851. https://onlinelibrary.wiley.com/doi/10.1002/anie.202103268.
- ↑ Dong, Wen; Wang, Chao; Ouyang, Yan; Liao, Dai-Zheng (March 2009). "Supramolecular Complexes Based on [M(CN) 8 4- (M = Mo, W) and Aliphatic Amine Cu II Tectons"] (in en). Zeitschrift für anorganische und allgemeine Chemie 635 (3): 544–548. doi:10.1002/zaac.200801254. https://onlinelibrary.wiley.com/doi/10.1002/zaac.200801254.
- ↑ Kuchár, J.; Miklošová, M.; Černák, J.; Falvello, L.R. (August 2014). "Tetracyanidopalladates of Cu(II) with 2-aminoethylpyridines as blocking ligands: The role of the 2-aminoethyl arm position on the pyridine ring" (in en). Journal of Molecular Structure 1072: 94–102. doi:10.1016/j.molstruc.2014.04.061. https://linkinghub.elsevier.com/retrieve/pii/S0022286014004281.
- ↑ 15.0 15.1 15.2 15.3 Arlt, Sören; Harloff, Jörg; Schulz, Axel; Stoffers, Alrik; Villinger, Alexander (5 December 2016). "Cyanido Antimonate(III) and Bismuthate(III) Anions". Inorganic Chemistry 55 (23): 12321–12328. doi:10.1021/acs.inorgchem.6b02174.
- ↑ Birk, Francisco J.; Pinkowicz, Dawid; Dunbar, Kim R. (2016-09-12). "The Heptacyanotungstate(IV) Anion: A New 5 d Transition‐Metal Member of the Rare Heptacyanometallate Family of Anions" (in en). Angewandte Chemie International Edition 55 (38): 11368–11371. doi:10.1002/anie.201602949. ISSN 1433-7851. https://onlinelibrary.wiley.com/doi/10.1002/anie.201602949.
- ↑ 17.0 17.1 17.2 Kobylarczyk, Jedrzej; Pinkowicz, Dawid; Srebro-Hooper, Monika; Hooper, James; Podgajny, Robert (2019-02-06). "Anion-π Architectures of HAT(CN) 6 and 5d Polycyanidometalates: [W(CN) 8 3– , [Re(CN) 7 ] 3– , and [Pt(CN) 6 ] 2–"] (in en). Crystal Growth & Design 19 (2): 1215–1225. doi:10.1021/acs.cgd.8b01653. ISSN 1528-7483. https://pubs.acs.org/doi/10.1021/acs.cgd.8b01653.
- ↑ Korkmaz, Şengül Aslan; Karadağ, Ahmet; Aydın, Ali; Yerli, Yusuf; Soylu, Mustafa Serkan (November 2016). "Binuclear cyanido complexes containing [Pt(CN)42− building block: Synthesis, crystal structures, magnetic properties and anticancer activities"] (in en). Inorganica Chimica Acta 453: 154–168. doi:10.1016/j.ica.2016.08.002. https://linkinghub.elsevier.com/retrieve/pii/S0020169316304431.
- ↑ Matsushita, Nobuyuki; Noguchi, Wataru; Tanaka, Rikako (2017-03-28). "Potassium tetracyanidoaurate(III) monohydrate: a redetermination". IUCrData 2 (3): x170382. doi:10.1107/S2414314617003820. ISSN 2414-3146. https://scripts.iucr.org/cgi-bin/paper?S2414314617003820.
- ↑ Fehlhammer, W. P. Fritz, M., "Emergence of a CNH and Cyano Complex Based Organometallic Chemistry", Chemical Reviews, 1993, volume 93, pp. 1243-80.doi:10.1021/cr00019a016
 |