Chemistry:Cyclopentadienylvanadium tetracarbonyl
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Appearance | orange solid |
| Density | 1.56 g/cm3 |
| Boiling point | sublimes |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H311, H315, H319, H330, H335 | |
| P260, P261, P264, P270, P271, P280, P284, P301+310, P302+352, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P332+313, P337+313, P361, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
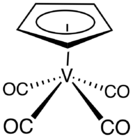
Cyclopentadienylvanadium tetracarbonyl is the organovanadium compound with the formula (C5H5)V(CO)4. An orange, diamagnetic solid, it is the principal cyclopentadienyl carbonyl of vanadium. It can be prepared by heating a solution of vanadocene under high pressure of carbon monoxide. As confirmed by X-ray crystallography, the coordination sphere of vanadium consists of η5-cyclopentadienyl and four carbonyl ligands. The molecule is a four-legged piano stool complex.[1] The compound is soluble in common organic solvents.[2] The compound has no commercial applications.
Reactions
Reduction with sodium amalgam gives the dianion of the tricarbonyl:
- CpV(CO)4 + 2 Na → Na2CpV(CO)3 + CO
Protonation of this salt gives Cp2V2(CO)5.[3]
Heating a mixture of cycloheptatriene and cyclopentadienylvanadium tetracarbonyl gives (cycloheptatrienyl)(cyclopentadienyl)vanadium ("trovacene").[4]
References
- ↑ Wilford, J.B.; Whitla, A.; Powell, H.M. (1967). "The Crystal and Molecular Structure of π-Cyclopentadienylvanadium Tetracarbonyl". Journal of Organometallic Chemistry 8 (3): 495–502. doi:10.1016/S0022-328X(00)83671-2.
- ↑ King, R.B.; Stone, F.G.A (1963). Cyclopentadienyl Metal Carbonyls and Some Derivatives. 7. 99–115. doi:10.1002/9780470132388.ch31. ISBN 9780470132388.
- ↑ Fischer, Ernst Otto; Schneider, Robert J. J. (1970). "Über Aromatenkomplexe von Metallen, CXIV. Darstellung und Reaktionen von Dicyclopentadienyl‐divanadin‐pentacarbonyl, (C 5 H 5 ) 2 V 2 (CO) 5". Chemische Berichte 103 (11): 3684–3695. doi:10.1002/cber.19701031133.
- ↑ King, R. B.; Stone, F. G. A. (1959). "π-Cylopentadienyl-π-Cycloheptatrienyl Vanadium". Journal of the American Chemical Society 81 (19): 5263–5264. doi:10.1021/ja01528a063.
 |
