Chemistry:Dammarane
From HandWiki
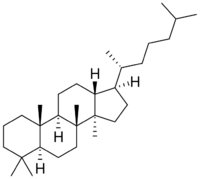
| |
| Names | |
|---|---|
| IUPAC name
Dammarane[1]
| |
| Systematic IUPAC name
(1R,3aR,3bR,5aS,9aS,9bR,11aR)-1-[(2R)-6-Methylheptan-2-yl]-3a,3b,6,6,9a-pentamethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | C102963 |
PubChem CID
|
|
| |
| |
| Properties | |
| C30H54 | |
| Molar mass | 414.75 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Dammarane is a tetracyclic triterpene found in sapogenins (forming triterpenoid saponins) like those of ginseng (ginsenosides: panaxatriol and protopanaxadiol). Compounds of the series were first isolated from and named after dammar resin, a natural resin from the tropical trees of the Dipterocarp family.[2] [3]
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 1535. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Mills J.S.; Werner A.E.A. (1955). "The Chemistry of Dammar Resin". Journal of the Chemical Society: 3132–40. doi:10.1039/jr9550003132.
- ↑ Mills J.S. (1956) "The Constitution of the Neutral, Tetracyclic Triterpenes of Dammar Resin" Journal of the Chemical Society 2196-2202
External links
 |
