Chemistry:Diosgenin
| |
| |
| Names | |
|---|---|
| IUPAC name
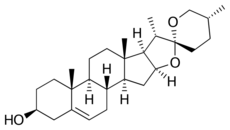
(25R)-Spirost-5-en-3β-ol
| |
| Systematic IUPAC name
(2S,2′R,4aR,4bS,5′R,6aS,6bR,7S,9aS,10aS,10bS)-4′,4a,6a,7-Tetramethyl-1,2,3,4,4a,4b,5,6,6a,6b,7,9a,10,10a,10b,11-hexadecahydrospiro[naphtho[2′,1′:4,5]indeno[2,1-b]furan-8,2′-oxan]-2-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H42O3 | |
| Molar mass | 414.630 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of Dioscorea wild yam species, such as the Kokoro. The sugar-free (aglycone) product of such hydrolysis, diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products.
Sources
It is present in detectable amounts in Costus speciosus, Smilax menispermoidea, Helicteres isora, species of Paris, Aletris, Trigonella, and Trillium, and in extractable amounts from many species of Dioscorea – D. althaeoides, D. colletti, D. composita,[1] D. floribunda, D. futschauensis, D. gracillima, D. hispida, D. hypoglauca, D. mexicana,[2] D. nipponica, D. panthaica, D. parviflora, D. septemloba, and D. zingiberensis.[3]
Industrial uses
Diosgenin is a chemical precursor for several hormones, starting with the Marker degradation process, which includes synthesis of progesterone.[4] The process was used in the early manufacturing of combined oral contraceptive pills.[5] Diosgenin in dietary supplements is not a physiological precursor to estradiol or progesterone, and the use of such products as wild yam has no hormonal activity in the human body.[6]
See also
References
- ↑ {{citation | mode = cs1 | title = Dioscorea composita | work = Germplasm Resources Information Network (GRIN) | url = https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?14190 | publisher = [[Organization:Agricultural Research ServAgricultural Research Service (ARS), United States Department of Agriculture (USDA) | access-date = 2008-09-14 }}
- ↑ {{citation | mode = cs1 | title = Dioscorea mexicana | work = Germplasm Resources Information Network (GRIN) | url = https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?14226 | publisher = [[Organization:Agricultural Research ServAgricultural Research Service (ARS), United States Department of Agriculture (USDA) | access-date = 2008-09-14 }}
- ↑ "2950 Diosgenin". http://tcm.cambridgesoft.com/tcm/showcompound.asp?monograph=2950&formgroup=basenp_form_group&dbname=TCM&formmode=edit.[yes|permanent dead link|dead link}}]
- ↑ "Sterols. CXII. Sapogenins. XLI. The Preparation of Trillin and its Conversion to Progesterone". J. Am. Chem. Soc. 62 (12): 3349–3350. 1940. doi:10.1021/ja01869a023.
- ↑ Djerassi C (December 1992). "Steroid research at Syntex: "the pill" and cortisone". Steroids 57 (12): 631–41. doi:10.1016/0039-128X(92)90016-3. PMID 1481227.
- ↑ Medigović I, Ristić N, Živanović J, Šošić-Jurjević B, Filipović B, Milošević V, Nestorović N “Diosgenin does not express estrogenic activity: a uterotrophic assay” Can J Physiol Pharmacol. 2014 Apr;92(4):292-8. doi: 10.1139/cjpp-2013-0419. Epub 2014 Feb 5. PMID: 24708211
External links
- Diosgenin at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
