Chemistry:Danavorexton
From HandWiki
Short description: Chemical compound
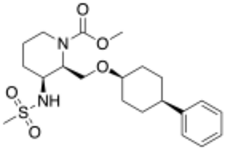 | |
| Clinical data | |
|---|---|
| Other names | TAK-925 |
| Routes of administration | Intravenous[1][2] |
| Drug class | Orexin receptor agonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C21H32N2O5S |
| Molar mass | 424.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Danavorexton (developmental code name TAK-925) is a selective orexin 2 receptor agonist.[1] It is a small-molecule compound and is administered intravenously.[1][2] The compound was found to dose-dependently produce wakefulness to a similar degree as modafinil in a phase 1 clinical trial.[1][3] As of March 2021, danavorexton is under development for the treatment of narcolepsy, idiopathic hypersomnia, and sleep apnea.[2][1][4] It is related to another orexin receptor agonist, firazorexton (TAK-994), the development of which was discontinued for safety reasons in October 2021.[1][5]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Hypocretins (orexins): The ultimate translational neuropeptides". J Intern Med 291 (5): 533–556. January 2022. doi:10.1111/joim.13406. PMID 35043499.
- ↑ 2.0 2.1 2.2 "Danavorexton - Takeda". Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800050631.
- ↑ Evans, R., Hazel, J., Faessel, H., Wu, J., Hang, Y., Alexander, R., ... & Hartman, D. (2019). Results of a phase 1, 4-period crossover, placebo-controlled, randomized, single dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-925, a novel orexin 2 receptor agonist, in sleep-deprived healthy adults, utilizing modafinil as an active comparator. Sleep Medicine, 64, S106. https://scholar.google.com/scholar?cluster=10933819770107034612
- ↑ "A Phase 1 single ascending dose study of a novel orexin 2 receptor agonist, TAK-925, in healthy volunteers (HV) and subjects with narcolepsy type 1 (NT1) to assess safety, tolerability, pharmacokinetics, and pharmacodynamic outcomes". Sleep Medicine 64: S105–S106. December 2019. doi:10.1016/j.sleep.2019.11.290.
- ↑ "Takeda flashes red light on 'breakthrough' narcolepsy drug after PhII trials turned up mysterious safety signal". Endpoints News. 6 October 2021. https://endpts.com/takeda-flashes-red-light-on-narcolepsy-drug-after-phii-trials-turned-up-mysterious-safety-signal/.
External links
 |

