Chemistry:Defactinib
From HandWiki
Short description: Pharmaceutical
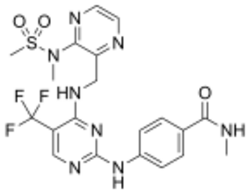 | |
 Skeletal formula and ball-and-stick model of defactinib | |
| Clinical data | |
|---|---|
| Other names | PF-04554878, VS-6063 |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C20H21F3N8O3S |
| Molar mass | 510.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Defactinib (INN, codenamed VS-6063) is an inhibitor of PTK2, also known as focal adhesion kinase (FAK), Pyk2, and MELK which was developed by Pfizer and licensed to Verastem Oncology as a potential treatment for solid tumors.
Development for mesothelioma was discontinued in 2015 due to lack of efficacy in a placebo-controlled phase II trial.[1][2] Subsequent research in patients with specific NF2 mutations also found limited activity.[3]
As of 2022, it remains in trials in combination with other medications for other types of cancer.
References
- ↑ Nathan VL (2015-09-28). "Verastem stops cancer therapy development; shares plunge" (Press release). Reuters. Retrieved 2022-06-05.
- ↑ "Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND-A Double-Blind, Randomized, Phase II Study". Journal of Clinical Oncology 37 (10): 790–798. April 2019. doi:10.1200/JCO.2018.79.0543. PMID 30785827.
- ↑ "A phase 2 study of defactinib (VS-6063) in patients with NF2 altered tumors: Results from NCI-match (EAY131) subprotocol U.". Journal of Clinical Oncology (American Society of Clinical Oncology (ASCO)) 39 (15_suppl): 3087. 2021-05-20. doi:10.1200/jco.2021.39.15_suppl.3087. ISSN 0732-183X.
 |

