Chemistry:Delsoline
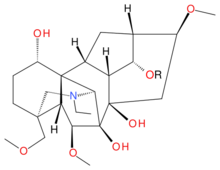 Delsoline (R = CH3)
| |
| Names | |
|---|---|
| IUPAC name
(1α,6β,14α,16β)-20-Ethyl-6,16-dimethoxy-4-(methoxymethyl)aconitane-1,7,8,14-tetrol
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C25H41NO7 | |
| Molar mass | 467.603 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Delsoline and delcosine are two closely related naturally occurring diterpene alkaloids first isolated from Delphinium consolida. They occur widely in the Ranunculaceae plant family. The polycyclic ring system containing nineteen carbon atoms and one nitrogen atom in these compounds is the same as in aconitine and this is reflected in their preferred IUPAC name.
History

Delsoline and delcosine were named in 1924 by Markwood, who isolated these alkaloids from Delphinium consolida, but their structures were in doubt[1] until established in 1963[2] and later confirmed by the X-ray crystallography of delsoline.[3][4] There are many other known diterpene alkaloids, some of which differ from these in only minor ways. Thus while delsoline (R=CH3) is a methylated derivative of delcosine (R=H), it is also an isomeric methyl derivative of gigactonine.[5]
Synthesis
Although individual members of this class of alkaloids have been extensively studied, their chemical complexity has limited the number which have been individually synthesised. Similarly, their full biosynthetic pathway is only known in outline in most cases.[6]
Natural occurrence
The compounds co-occur in Aconitum variegatum[7] and many species of Delphinium[8] including Delphinium ajacis[9] and other Consolida species in the Ranunculaceae plant family.[10]
Biochemistry
Compounds related to aconitine are widely studied for their properties in biological systems and these have been reviewed.[10] For example, delsoline has been reported be one of the active components in traditional Chinese medicinal plants.[11]
References
- ↑ Marion, Léo; Edwards, O. E. (1947). "The Alkaloids of Delphinium Consolida L.1". Journal of the American Chemical Society 69 (8): 2010–2014. doi:10.1021/ja01200a055. PMID 20255295.
- ↑ Marion, L. (1963). "The highly-oxygenated diterpenoid alkaloids". Pure and Applied Chemistry 6 (4): 621–634. doi:10.1351/pac196306040621.
- ↑ Pelletier, S.W.; Keith, L.H. (1970). Chapter 1 Diterpene Alkaloids from Aconitum, Delphinium, and Garry a Species: The C19-Diterpene Alkaloids. The Alkaloids: Chemistry and Physiology. 12. pp. 1–134. doi:10.1016/S1876-0813(08)60100-1. ISBN 9780124695122.
- ↑ Joshi, Balawant S.; Desai, Haridutt K.; Pelletier, S. William; Newton, M. Gary (1992). "Crystal and molecular structure of delsoline". Journal of Crystallographic and Spectroscopic Research 22 (4): 477–483. doi:10.1007/BF01195410.
- ↑ Sakai, S.; Shinma, N.; Hasegawa, S.; Okamoto, T. (1978). "On the Alkaloids of Aconitum gigas Lev. et Van. and the structure of a New Base, Gigactonine". Yakugaku Zasshi 98 (10): 1376–1384. doi:10.1248/yakushi1947.98.10_1376. https://www.jstage.jst.go.jp/article/yakushi1947/98/10/98_10_1376/_pdf.
- ↑ Cherney, Emily C.; Baran, Phil S. (2011). "Terpenoid-Alkaloids: Their Biosynthetic Twist of Fate and Total Synthesis". Israel Journal of Chemistry 51 (3–4): 391–405. doi:10.1002/ijch.201100005. PMID 26207071.
- ↑ Díaz, Jesús G.; Ruiza, Juan García; Herz, Werner (April 2005). "Norditerpene and diterpene alkaloids from Aconitum variegatum". Phytochemistry 66 (7): 837–46. doi:10.1016/j.phytochem.2005.01.019. PMID 15797610.
- ↑ Yin, Tianpeng; Cai, Le; Ding, Zhongtao (2020). "An overview of the chemical constituents from the genus Delphinium reported in the last four decades". RSC Advances 10 (23): 13669–13686. doi:10.1039/D0RA00813C. PMID 35492993. Bibcode: 2020RSCAd..1013669Y.
- ↑ Pelletier, S. William; Bhandaru, Sudhakar; Desai, Haridutt K.; Ross, Samir A.; Sayed, Hanna M. (1992). "Two New Norditerpenoid Alkaloids from the Roots of Delphinium ajacis". Journal of Natural Products 55 (6): 736–743. doi:10.1021/np50084a005.
- ↑ 10.0 10.1 Yin, Tianpeng; Cai, Le; Ding, Zhongtao (2020). "A systematic review on the chemical constituents of the genus Consolida (Ranunculaceae) and their biological activities". RSC Advances 10 (58): 35072–35089. doi:10.1039/D0RA06811J. PMID 35515663. Bibcode: 2020RSCAd..1035072Y.
- ↑ Han, Gui-Qiu; Chen, Ya-Yan; Liang, Xiao-Tian (1988). Chapter 3 Distribution of Alkaloids in Traditional Chinese Medicinal Plants. The Alkaloids: Chemistry and Pharmacology. 32. pp. 241–270. doi:10.1016/S0099-9598(08)60217-5. ISBN 9780124695320.
See also
 |
