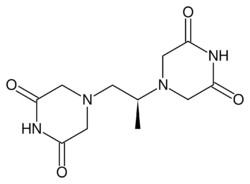
Chemistry:Dexrazoxane
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H16N4O4 |
| Molar mass | 268.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Dexrazoxane hydrochloride (Zinecard, Cardioxane) is a cardioprotective agent. It was discovered by Eugene Herman in 1972. The IV administration of dexrazoxane is in acidic condition with HCl adjusting the pH.[1]
Uses
Dexrazoxane has been used to protect the heart against the cardiotoxic side effects of chemotherapeutic drugs such as anthracyclines,[2] such as daunorubicin or doxorubicin or other chemotherapeutic agents.[3] However, in July 2011 the European Medicines Agency (EMA) released a statement restricting use only in adult patients with cancer who have received > 300 mg/m2 doxorubicin or > 540 mg/m2 epirubicin and general approval for use for cardioprotection.[4][5] That showed a possibly higher rate of secondary malignancies and acute myelogenous leukemia in pediatric patients treated for different cancers with both dexrazoxane and other chemotherapeutic agents that are associated with secondary malignancies.[6] On July 19, 2017, based on evaluation of the currently available data the European Commission issued an EU-wide legally binding decision to implement the recommendations of the Committee for Medicinal Products for Human Use (CHMP) on dexrazoxane and lifted its 2011-contraindication for primary prevention of anthracycline-induced cardiotoxicity with dexrazoxane in children and adolescents where high doses (≥ 300 mg/m3) of anthracyclines are anticipated.
Dexrazoxane was designated by the US FDA as an orphan drug for "prevention of cardiomyopathy for children and adults 0 through 16 years of age treated with anthracyclines".[7] This decision allows virtually all children to receive dexrazoxane starting with the first dose of anthracycline at the discretion of the treating provider. The label change by the agency announcing dexrazoxane as an approved cardio-oncology protectant has been followed by a review by the agency.[8] Currently, the only FDA and EMA approved cardioprotective treatment for anthracycline cardioprotection is dexrazoxane, which provides effective primary cardioprotection against anthracycline-induced cardiotoxicity without reducing anthracycline activity and without enhancing secondary malignancies.[9]
The United States Food and Drug Administration has also approved a dexrazoxane for use as a treatment of extravasation resulting from IV anthracycline chemotherapy.[10][11] Extravasation is an adverse event in which chemotherapies containing anthracylines leak out of the blood vessel and necrotize the surrounding tissue.
Mechanism
As a derivative of EDTA, dexrazoxane chelates iron and thus reduces the number of metal ions complexed with anthracycline and, consequently, decrease the formation of superoxide radicals.[12] The exact chelation mechanism is unknown, but it has been postulated that dexrazoxane can be converted into ring-opened form intracellularly and interfere with iron-mediated free radical generation that is in part thought to be responsible for anthryacycline induced cardiomyopathy.[13] It was speculated that dexrazoxane could be used for further investigation to synthesize new antimalarial drugs.[14]
References
- ↑ "Zinecard (Dexrazoxane): Side Effects, Interactions, Warning, Dosage & Uses". http://www.rxlist.com/zinecard-drug.htm.
- ↑ "The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia". The New England Journal of Medicine 351 (2): 145–53. July 2004. doi:10.1056/NEJMoa035153. PMID 15247354.
- ↑ "Effects of dexrazoxane and amifostine on evolution of Doxorubicin cardiomyopathy in vivo". Experimental Biology and Medicine 232 (11): 1414–24. December 2007. doi:10.3181/0705-RM-138. PMID 18040065.
- ↑ "Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease". Journal of Clinical Oncology 25 (5): 493–500. February 2007. doi:10.1200/JCO.2005.02.3879. PMID 17290056.
- ↑ "Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children's oncology group". Leukemia 24 (2): 355–70. February 2010. doi:10.1038/leu.2009.261. PMID 20016527.
- ↑ "FDA Statement on Dexrazoxane". Archived by the Wayback Machine. 20 July 2011. https://www.fda.gov/Drugs/DrugSafety/ucm263729.htm.
- ↑ "Orphan drug designations and approvals". https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=441314.
- ↑ "Cardioxane". 2018-09-17. https://www.ema.europa.eu/en/medicines/human/referrals/cardioxane.
- ↑ "Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling". Future Oncology 14 (25): 2663–2676. October 2018. doi:10.2217/fon-2018-0210. PMID 29747541.
- ↑ "NDA 22-025 TOTECT™ (DEXRAZOXANE) FOR INJECTION | Totect™ Package Insert". Archived by the Wayback Machine. https://www.fda.gov/cder/foi/label/2007/022025lbl.pdf.
- ↑ "Dexrazoxane (Totect): FDA review and approval for the treatment of accidental extravasation following intravenous anthracycline chemotherapy". The Oncologist 13 (4): 445–50. April 2008. doi:10.1634/theoncologist.2007-0247. PMID 18448560.
- ↑ "Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity". Expert Review of Cardiovascular Therapy 6 (10): 1311–7. November 2008. doi:10.1586/14779072.6.10.1311. PMID 19018683.
- ↑ "ZINECARD- dexrazoxane hydrochloride injection, powder, lyophilized, for solution". Pharmacia and Upjohn Company LLC. http://labeling.pfizer.com/ShowLabeling.aspx?id=514.
- ↑ "Plasmodium falciparum and Plasmodium yoelii: effect of the iron chelation prodrug dexrazoxane on in vitro cultures". Experimental Parasitology 91 (2): 105–14. February 1999. doi:10.1006/expr.1998.4371. PMID 9990337.
External links
- "Dexrazoxane". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dexrazoxane.
- "Dexrazoxane hydrochloride". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dexrazoxane%20hydrochloride.
 |
