Chemistry:Diamidophosphate
| |
| Names | |
|---|---|
| IUPAC name
diaminophosphinate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H4N2O2P | |
| Molar mass | 95.018 g·mol−1 |
| Related compounds | |
Other anions
|
Thiophosphordiamidic acid |
Other cations
|
Phosphordiamidic acid |
Related
|
phosphorotriamide phosphoramidic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
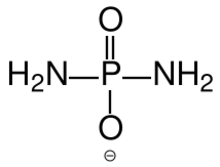
Diamidophosphate (DAP) is the simplest phosphorodiamidate ion, with formula PO2(NH2)2−. It is a phosphorylating ion and was first used for phosphorylation of sugars in aqueous medium.[1] DAP has attracted interest in the area of primordial chemistry.[2]
Salts
Several salts of the formula MPO2(NH2)2(H2O)x are known.[3]
- The sodium salt can be made by base hydrolysis of phenyl phosphorodiamidate.[4] It crystallises as a hexahydrate. It can be dehydrated.
- The silver salt AgPO2(NH2)2 can react using double decomposition with bromides forming other salts.
- The potassium diamidophosphate salt KPO2(NH2)2 is also known.
- Phosphorodiamidic acid crystallizes as a trihydrate.[4]
Reactions
Heating anhydrous sodium diamidophosphate causes polmerization:[3]
- At 160 °C, Na2P2O4(NH)(NH2)2, Na3P3O6(NH)2(NH2)2, Na4P4O8(NH)3(NH2)2, Na5P5O10(NH)4(NH2)2 and Na6P6O12(NH)5(NH2)2 are produced. These substances contain P-N-P backbones. These can be separated by paper chromatography.
- At 200 °C the hexa-phosphate is produced.
- At 250 °C the typical chain length is 18.
Heating hydrated salts induces loss of ammonia to form oligophosphates and polyphosphates.[3]
Diamidophosphate inhibits urease enzymes by blocking up the active site, binding to two nickel centers. Diamidophosphate mimics the urea hydrolysis intermediate.[5]
Diamidophosphate is tribasic, and the amine groups may also lose hydrogen to form more metallic salts. With silver, further reactions can yield explosive salts: tetrasilver orthodiamidophosphate (AgO)3P(NH2)NHAg, and pentasilver orthodiamidophosphate (AgO)3P(NHAg)2.[6]
Organic esters and amides
thumb|left|[[Phenyl phosphorodiamidate, an inhibitor of urease, is a controlled release fertilizers.[7]]] Numerous organic derivatives are known. One example is phenyl phosphorodiamidate.[8]
Reactions with nucleosides
DAP phosphorylates deoxynucleosides (the building blocks of DNA, and at the same time initiates polymerization to make DNA.[9] DAP facilitates the synthesis of larger RNA sequences (ribozymes) from smaller RNA strands.[10] Other nitrogenous derivatives of phosphorus derivatives have also been proposed in this context in a review article.[11]
See also
References
- ↑ Krishnamurthy, Ramanarayanan; Guntha, Sreenivasulu; Eschenmoser, Albert (4 July 2000). "Regioselective α-Phosphorylation of Aldoses in Aqueous Solution" (in en). Angewandte Chemie International Edition 39 (13): 2281–2285. doi:10.1002/1521-3773(20000703)39:13<2281::AID-ANIE2281>3.0.CO;2-2. ISSN 1521-3773. PMID 10941064.
- ↑ Gibard, Clémentine; Bhowmik, Subhendu; Karki, Megha; Kim, Eun-Kyong; Krishnamurthy, Ramanarayanan (2018). "Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions". Nature Chemistry 10 (2): 212–217. doi:10.1038/nchem.2878. PMID 29359747.
- ↑ 3.0 3.1 3.2 Klement, R.; Biberacher, G. (May 1956). "Das thermische Verhalten von Natriumdiamidophosphat, Darstellung von kondensierten Imidophosphaten". Zeitschrift für Anorganische und Allgemeine Chemie 285 (1–2): 74–85. doi:10.1002/zaac.19562850109.
- ↑ 4.0 4.1 Coggins, Adam J.; Powner, Matthew W. (10 October 2016). "Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis Supplementary Information Compound 8" (in en). Nature Chemistry 9 (4): 310–317. doi:10.1038/nchem.2624. ISSN 1755-4349. PMID 28338685. Bibcode: 2017NatCh...9..310C. http://discovery.ucl.ac.uk/1520873/1/FINAL_ACCEPTED_PEP_NAT_CHEM_MANUSCRIPT.pdf.
- ↑ Deborah Zamble; Rowińska-Żyrek, Magdalena; Kozlowski, Henryk (2017) (in en). The Biological Chemistry of Nickel. Royal Society of Chemistry. pp. 73–74, 83. ISBN 9781788010580. https://books.google.com/books?id=UmwoDwAAQBAJ&pg=PA73.
- ↑ Bretherick, L. (2016) (in en). Bretherick's Handbook of Reactive Chemical Hazards. Elsevier. p. 19. ISBN 9781483162508. https://books.google.com/books?id=4_PJCgAAQBAJ&pg=PA19.
- ↑ Pan, Baobao; Lam, Shu Kee; Mosier, Arvin; Luo, Yiqi; Chen, Deli (2016). "Ammonia Volatilization from Synthetic Fertilizers and its Mitigation Strategies: A Global Synthesis". Agriculture, Ecosystems & Environment 232: 283–289. doi:10.1016/j.agee.2016.08.019.
- ↑ Kiss, S.; Simihaian, M. (2013) (in en). Improving Efficiency of Urea Fertilizers by Inhibition of Soil Urease Activity. Springer Science & Business Media. pp. 105–108. ISBN 9789401718431. https://books.google.com/books?id=Tp5fBgAAQBAJ&pg=PA105.
- ↑ Krishnamurthy, Ramanarayanan; Jiménez, Eddy I.; Gibard, Clémentine (2020). "Prebiotic Phosphorylation and Concomitant Oligomerization of Deoxynucleosides to form DNA" (in en). Angewandte Chemie International Edition 60 (19): 10775–10783. doi:10.1002/anie.202015910. ISSN 1521-3773. PMID 33325148. https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202015910.
- ↑ Song, Emilie Yeonwha; Jiménez, Eddy Ivanhoe; Lin, Huacan; Vay, Kristian Le; Krishnamurthy, Ramanarayanan; Mutschler, Hannes (2020). "Prebiotically Plausible RNA Activation Compatible with Ribozyme-Catalyzed Ligation" (in en). Angewandte Chemie International Edition 60 (6): 2952–2957. doi:10.1002/anie.202010918. ISSN 1521-3773. PMID 33128282.
- ↑ Karki, Megha; Gibard, Clémentine; Bhowmik, Subhendu; Krishnamurthy, Ramanarayanan (2017-07-29). "Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water" (in en). Life 7 (3): 32. doi:10.3390/life7030032. PMID 28758921. Bibcode: 2017Life....7...32K.
Other reading
- H. N. Stokes (1894). "On Diamidoorthophosphoric and Diamidotrihydroxyphosphoric Acids". American Chemical Journal 16 (2): 123.
 |

