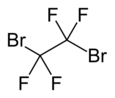
Chemistry:Dibromotetrafluoroethane
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dibromo-1,1,2,2-tetrafluoroethane | |||
| Other names
R-114B2,[1] Halon 2402
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2Br2F4 | |||
| Appearance | Colorless liquid | ||
| Density | 2180 kg/m3 at 20°C | ||
| Boiling point | 47.3 °C (117.1 °F; 320.4 K) | ||
| not soluble in water | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H420 | |||
| P502 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
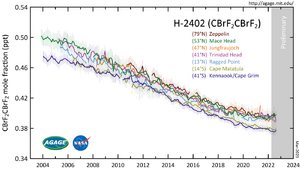
1,2-Dibromotetrafluoroethane (C2Br2F4) is a haloalkane. It is also known under codenames R-114B2 and Halon 2402. It is a colorless liquid with a boiling point of 47.2 °C. R-114B2 is occasionally used in fire suppression systems. It is highly volatile, passes through soil to air, and can be detected in the parts-per-quadrillion range.[2]
Since July 1, 1994, the Montreal Protocol required all nations or parties that are a party to it to eliminate the production, consumption, and trade of ozone-depleting substances (ODS). Dibromotetrafluoroethane's high ozone-depleting potential (ODP) caused it to be identified as an ODS. Dibromotetrafluoroethane has been prohibited in Canada since July 1, 1994, "except for essential uses or for use as analytical standards".[3]
On November 8, 2008, an accident aboard Russian submarine K-152 Nerpa involving the unintentional activation of a fire suppressant system loaded with R-114B2 resulted in the death of 20 people.[4]
Notes
- ↑ "Chemical datasheet for dibromotetrafluoroethane". Cameo Chemicals. National Oceanic and Atmospheric Administration. http://cameochemicals.noaa.gov/chemical/16320. Retrieved November 18, 2008.
- ↑ Patent #4725551 and Patent #6817227
- ↑ "ARCHIVED - Environment and Climate Change Canada - Pollution and Waste - Dibromotetrafluoroethane". Environment and Climate Change Canada. http://www.ec.gc.ca/Toxiques-toxics/Default.asp?lang=En&n=98E80CC6-1&xml=BCD35FA1-FC4D-40E1-B4AB-D312F7CCF2D5.
- ↑ Eschel, David (November 11, 2008). "Fire on Board the Russian Navy Akula II Nuclear Submarine kills Twenty Russian Sailors". Defense Update. http://www.defense-update.com/analysis/analysis_111108_russian_submarine_fire.html. Retrieved November 18, 2008.
 |