Chemistry:Dichlofluanid
From HandWiki
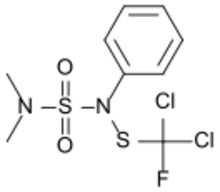
| |
| Names | |
|---|---|
| IUPAC name
N-{[Dichloro(fluoro)methyl]sulfanyl}-N′,N′-dimethyl-N-phenylsulfuric diamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H11Cl2FN2O2S2 | |
| Molar mass | 333.22 g·mol−1 |
| Density | 1.55 g/cm3 |
| Melting point | 105–106 °C (221–223 °F; 378–379 K) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2500 mg/kg (rat)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dichlofluanid (N-dichlorofluoromethylthio-N′, N′-dimethyl-N-phenylsulfamide) is a fungicide used to protect strawberries, grapes, berries, apples, pears and other fruit, vegetables and ornamental plants from diseases such as apple scab (Venturia inaequalis),[2] black spot, leather rot, gray mold, downy mildew and others caused by the fungi Botrytis, Alternaria, Sclerotinia, and Monilinia. It is also used to protect against diseases of fruit during storage,[citation needed] and as a wood preservative, often as part of a paint undercoat.[3]
Dichlofluanid was first marketed by Bayer Company in 1964, for use as a fungicide on plants.[3] Its trade names include Euparen and Elvaron.[1]
References
- ↑ 1.0 1.1 Zhou, X; Cao, S; Li, X; Xi, C; Ding, X; Xu, F; Hu, J; Chen, Z (2016). "Rapid Determination of Dichlofluanid Residues in Vegetables Using Dispersive-SPE Sample Preparation Combined with Gas Chromatography-Mass Spectrometry". Journal of Chromatographic Science 54 (5): 858–63. doi:10.1093/chromsci/bmw006. PMID 26921896.
- ↑ Matolcsy, György; Nádasy, Miklós; Andriska, Viktor, eds (1988). "5. Fungicides". Studies in Environmental Science: Volume 32 Pesticide chemistry. Amsterdam: Elsevier. pp. 341. ISBN 978-0-444-98903-1.
- ↑ 3.0 3.1 Unger, A; Schniewind, AP; Unger, W (2001). "7.3.9.1.: Dichlofluanid (N-Dichlorofluoromethylthio-N'-N'-dimethyl-N-phenylsulfamide)". Conservation of Wood Artifacts: A Handbook. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 217–218. ISBN 9783662063989. https://archive.org/details/conservationwood00unge.
External links
- Rocket NXT
- Hamwijk, C; Schouten, A; Foekema, E.M; Ravensberg, J.C; Collombon, M.T; Schmidt, K; Kugler, M (2005). "Monitoring of the booster biocide dichlofluanid in water and marine sediment of Greek marinas". Chemosphere 60 (9): 1316–1324. doi:10.1016/j.chemosphere.2005.01.072. PMID 16018903. Bibcode: 2005Chmsp..60.1316H.
- Dichlofluanid toxicity reports, review - hazard potential, risk
- Waliszewski, S. M; Waliszewski, K. N (1988). "GC determination of dichlofluanid (Euparen) residues and its metabolite dimethylphenylsulfamide (DMSA) in strawberries". Fresenius' Zeitschrift für Analytische Chemie 331 (5): 528–529. doi:10.1007/BF00467044.
- Directive 98/8/EC concerning the placing of biocidal products on the market, Assessment Report Dichlofluanid
- Ham, Norman S (1961). "Dichlorofluoromethanesulfenyl Chloride". Journal of the American Chemical Society 83 (3): 751–752. doi:10.1021/ja01464a052.
- T.R. Roberts, D.H. Hutson, Metabolic Pathways of agrochemicals. Part one: Herbicides and Plant Growth Regulators, Royal Society of Chemistry Publishers, London (1998)
 |

