Chemistry:Dichlororuthenium tricarbonyl dimer
From HandWiki
| |
| Names | |
|---|---|
| Other names
hexacarbonyldi-μ-chlorodichlorodiruthenium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6Cl4O6Ru2 | |
| Molar mass | 512.00 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
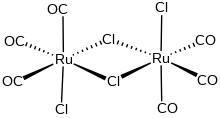
Dichlororuthenium tricarbonyl dimer is an organoruthenium compound with the formula [RuCl2(CO)3]2. A yellow solid, the molecule features a pair of octahedral Ru centers bridged by a pair of chloride ligands. The complex is a common starting material in ruthenium chemistry.[1]
Synthesis and reactions
Dichlororuthenium tricarbonyl dimer arises by the carbonylation of a hot solution of ruthenium trichloride in methoxyethanol.[2]
The complex exists in equilibrium with the polymer:
- n [RuCl2(CO)3]2 → [RuCl2(CO)2]2n + 2 CO
It reacts with potassium hydroxide in alcohol to give triruthenium dodecacarbonyl.
Dichlororuthenium tricarbonyl dimer reacts with glycine to give tricarbonylchloroglycinatoruthenium(II).[3]
References
- ↑ Haukka, Matti; Kiviaho, Jari; Ahlgren, Markku; Pakkanen, Tapani A. (1995). "Studies on Catalytically Active Ruthenium Carbonyl Bipyridine Systems. Synthesis and Structural Characterization of [Ru(bpy)(CO)2Cl2], [Ru(bpy)(CO)2Cl(C(O)OCH3)], [Ru(bpy)(CO)2Cl]2, and [Ru(bpy)(CO)2ClH] (Bpy = 2,2'-Bipyridine)". Organometallics 14 (2): 825–833. doi:10.1021/om00002a033.
- ↑ Fauré, Matthieu; Saccavini, Catherine; Lavigne, Guy (2004). Dodecacarbonyltriruthenium. Inorganic Syntheses. 34. p. 110. doi:10.1002/0471653683.ch3.
- ↑ Clark, James E.; Naughton, Patrick; Shurey, Sandra; Green, Colin J.; Johnson, Tony R.; Mann, Brian E.; Foresti, Roberta; Motterlini, Roberto (2003). "Cardioprotective Actions by a Water-Soluble Carbon Monoxide–Releasing Molecule". Circulation Research 93 (2): e2-8. doi:10.1161/01.RES.0000084381.86567.08. PMID 12842916.
 |

