Chemistry:Triruthenium dodecacarbonyl
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
cyclo-tris(tetracarbonylruthenium)
| |
| Other names
Ruthenium carbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12O12Ru3 | |
| Molar mass | 639.33 g/mol |
| Appearance | orange solid |
| Density | 2.48 g/cm3 |
| Melting point | 224 °C (435 °F; 497 K) |
| Boiling point | sublimes in vacuum |
| insoluble | |
| Solubility in organic solvents | soluble |
| Structure | |
| D3h cluster | |
| 0 D | |
| Hazards | |
| Main hazards | Toxic, CO Source |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Triiron dodecacarbonyl Triosmium dodecacarbonyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
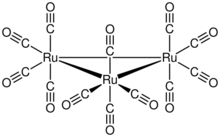
Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.
Structure and synthesis
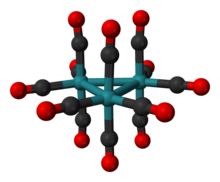
The cluster has D3h symmetry, consisting of an equilateral triangle of Ru atoms, each of which bears two axial and two equatorial CO ligands. The Ru-Ru distance is 284 pm.[1] Os3(CO)12 has the same structure. In Fe3(CO)12, two CO ligands are bridging, resulting in C2v symmetry. In solution, Ru
3(CO)
12 is fluxional as indicated by the observation of a single CO signal in the room temperature 13C NMR spectrum. The barrier is estimated at 20 kJ/mol[2]
Ru
3(CO)
12 is prepared by treating solutions of ruthenium trichloride with carbon monoxide in the presence of a base. Dichlororuthenium tricarbonyl dimer is an intermediate.[3][4] The stoichiometry of the reaction is uncertain, one possibility being the following:
- 6 RuCl3 + 33 CO + 18 CH3OH → 2 Ru3(CO)12 + 9 CO(OCH3)2 + 18 HCl
Reactions
The chemical properties of Ru3(CO)12 have been widely studied, and the cluster has been converted to hundreds of derivatives. High pressures of CO convert the cluster to the monomeric ruthenium pentacarbonyl, which reverts to the parent cluster upon standing.
- Ru3(CO)12 + 3 CO ⇌ 3 Ru(CO)5 Keq = 3.3 x 10−7 mol dm−3 at room temperature
The instability of Ru(CO)5 contrasts with the robustness of the corresponding Fe(CO)5. The condensation of Ru(CO)5 into Ru3(CO)12 proceeds via initial, rate-limiting loss of CO to give the unstable, coordinatively unsaturated species Ru(CO)4. This tetracarbonyl binds Ru(CO)5, initiating the condensation.[5]
Upon warming under a pressure of hydrogen, Ru3(CO)12 converts to the tetrahedral cluster H4Ru4(CO)12.[6] Ru3(CO)12 undergoes substitution reactions with Lewis bases:
- Ru3(CO)12 + n L → Ru3(CO)12-nLn + n CO (n = 1, 2, or 3)
where L is a tertiary phosphine or an isocyanide. It forms complexes with acenaphthylene.[7]
Ru
3(CO)
12 forms a variety of alkene complexes, some where the Ru3 core remains intact but often with fragmentation. Upon treatment with 1,5-cyclooctadiene gives the monoRu tricarbonyl derivative:[8]
- Ru
3(CO)
12 + 3 C
8H
12 → 3 Ru(C
8H
12)(CO)
3 + 3 CO
Ru-carbido clusters
At high temperatures, Ru3(CO)12 converts to a series of clusters that contain interstitial carbido ligands. These include Ru6C(CO)17 and Ru5C(CO)15. Anionic carbido clusters are also known, including [Ru5C(CO)14]2− and the bioctahedral cluster [Ru10C2(CO)24]2−.[9] Ru3(CO)12 -derived carbido compounds have been used to synthesize nanoparticles for catalysis. These particles consist of 6-7 atoms and thus are all surface, resulting in extraordinary activity.
References
- ↑ Slebodnick, C.; Zhao, J.; Angel, R.; Hanson, B. E.; Song, Y.; Liu, Z.; Hemley, R. J. (2004). "High Pressure Study of Ru3(CO)12 by X-ray Diffraction, Raman, and Infrared Spectroscopy". Inorg. Chem. 43 (17): 5245–5252. doi:10.1021/ic049617y. PMID 15310201.
- ↑ Farrugia, Louis J. (1997). "Dynamics and fluxionality in metal carbonyl clusters: Some old and new problems". Journal of the Chemical Society, Dalton Transactions (11): 1783–1792. doi:10.1039/A608514H.
- ↑ Bruce, M. I.; Jensen, C. M.; Jones, N. L. (1989). Dodecacarbonyltriruthenium, Ru3(CO)12. Inorganic Syntheses. 26. pp. 259–61. doi:10.1002/9780470132579.ch45.
- ↑ Fauré, Matthieu; Saccavini, Catherine; Lavigne, Guy (2004). Dodecacarbonyltriruthenium. Inorganic Syntheses. 34. p. 110. doi:10.1002/0471653683.ch3.
- ↑ Hastings, W. R.; Roussel, M. R.; Baird, M. C. "Mechanism of the conversion of [Ru(CO)5] into [Ru3(CO)12]" Journal of the Chemical Society, Dalton Transactions, 1990, pages 203-205. doi:10.1039/DT9900000203
- ↑ Bruce, M. I.; Williams, M. L. "Dodecacarbonyl(tetrahydrido)tetraruthenium, Ru4(μ-H)4(CO)12" Inorganic Syntheses, 1989, volume 26, pages 262-63. ISBN:0-471-50485-8.
- ↑ Motoyama, Yukihiro; Itonaga, Chikara; Ishida, Toshiki; Takasaki, Mikihiro; Nagashima, Hideo (2005). "Catalytic Reduction of Amides to Amines with Hydrosilanes Using a Triruthenium Cluster as the Catalyst". Organic Syntheses 82: 188. doi:10.15227/orgsyn.082.0188.
- ↑ Domingos, A. J. P.; Howell, J. A. S.; Johnson, B. F. G.; Lewis, J. (1990). "Reagents for the Synthesis of η‐Diene Complexes of Tricarbonnyliron and Tricarbonylruthenium". Inorganic Syntheses. 28. pp. 52–55. doi:10.1002/9780470132593.ch11. ISBN 9780471526193.
- ↑ Nicholls, J. N.; Vargas, M. D. "Carbido-Carbonyl Ruthenium Cluster Complexes" Inorganic Syntheses, 1989, volume 26, pages 280-85. doi:10.1002/9780470132579.ch49
 |

